Gallbladder Cancer Treatment (PDQ®)–Health Professional Version
General Information About Gallbladder Cancer
Incidence and Mortality
Estimated new cases and deaths from gallbladder (and other biliary) cancer in the United States in 2025:[1]
- New cases: 12,610.
- Deaths: 4,400.
Cancer that arises in the gallbladder is uncommon.
Clinical Features
The most common symptoms caused by gallbladder cancer are jaundice, pain, and fever.
Histopathology and Diagnostics
In patients whose superficial cancer (T1 or confined to the mucosa) is discovered on pathological examination of tissue after gallbladder removal for other reasons, the disease is often cured without further therapy. In patients who present with symptoms, the tumor is rarely diagnosed preoperatively.[2] In such cases, the tumor often cannot be removed completely by surgery and the disease cannot be cured, although palliative measures may be beneficial. For patients with T2 or greater disease, extended resection with partial hepatectomy and portal lymph node dissection may be an option.[3,4]
Other Prognostic Factors
Cholelithiasis is an associated finding in most cases, but less than 1% of patients with cholelithiasis develop this cancer.
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Chao TC, Greager JA: Primary carcinoma of the gallbladder. J Surg Oncol 46 (4): 215-21, 1991. [PUBMED Abstract]
- Shoup M, Fong Y: Surgical indications and extent of resection in gallbladder cancer. Surg Oncol Clin N Am 11 (4): 985-94, 2002. [PUBMED Abstract]
- Sasson AR, Hoffman JP, Ross E, et al.: Trimodality therapy for advanced gallbladder cancer. Am Surg 67 (3): 277-83; discussion 284, 2001. [PUBMED Abstract]
Cellular Classification of Gallbladder Cancer
Some histological types of gallbladder cancer have a better prognosis than others. Papillary carcinomas have the best prognosis. The histological types of gallbladder cancer include:[1]
- Carcinoma in situ.
- Biliary intraepithelial neoplasia, high grade.
- Intracystic papillary neoplasm with high-grade intraepithelial neoplasia.
- Mucinous cystic neoplasm with high-grade intraepithelial neoplasia.
- Adenocarcinoma.
- Adenocarcinoma, biliary type.
- Adenocarcinoma, intestinal type.
- Adenocarcinoma, gastric foveolar type.
- Mucinous adenocarcinoma.
- Clear cell adenocarcinoma.
- Signet-ring cell carcinoma.
- Squamous cell carcinoma.
- Adenosquamous carcinoma.
- Undifferentiated carcinoma.
- High-grade neuroendocrine carcinoma.
- Small cell neuroendocrine carcinoma.
- Mixed adenoneuroendocrine carcinoma.
- Intraductal papillary neoplasm with an associated invasive carcinoma.
- Mucinous cystic neoplasm with an associated invasive carcinoma.
References
- Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 303–9.
Stage Information for Gallbladder Cancer
AJCC Stage Groupings and TNM Definitions
The American Joint Committee on Cancer (AJCC) has designated staging by the TNM classification to define gallbladder cancer.[1]
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
| 0 | Tis, N0, M0 | Tis = Carcinoma in situ. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
| I | T1, N0, M0 | T1 = Tumor invades the lamina propria or muscular layer. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
| IIA | T2a, N0, M0 | T2a = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
| IIB | T2b, N0, M0 | T2b = Tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
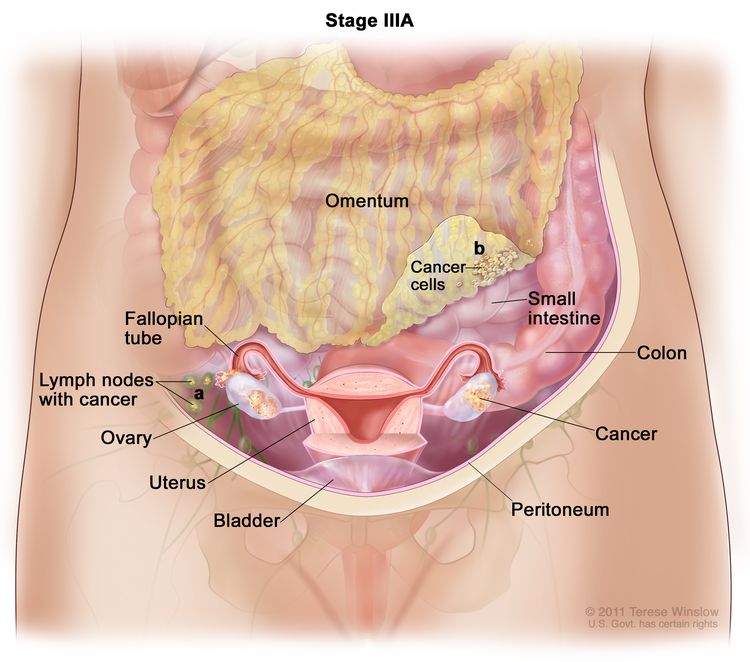
| IIIA | T3, N0, M0 | T3 = Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
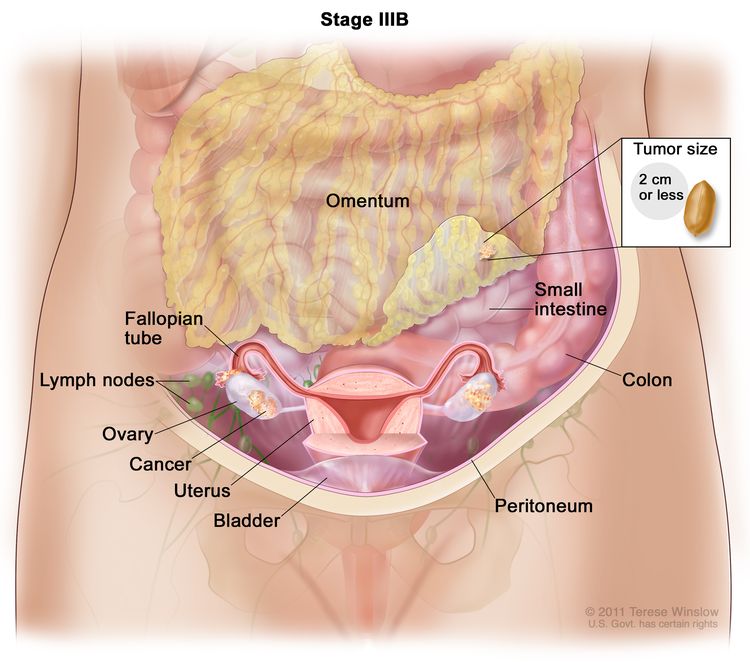
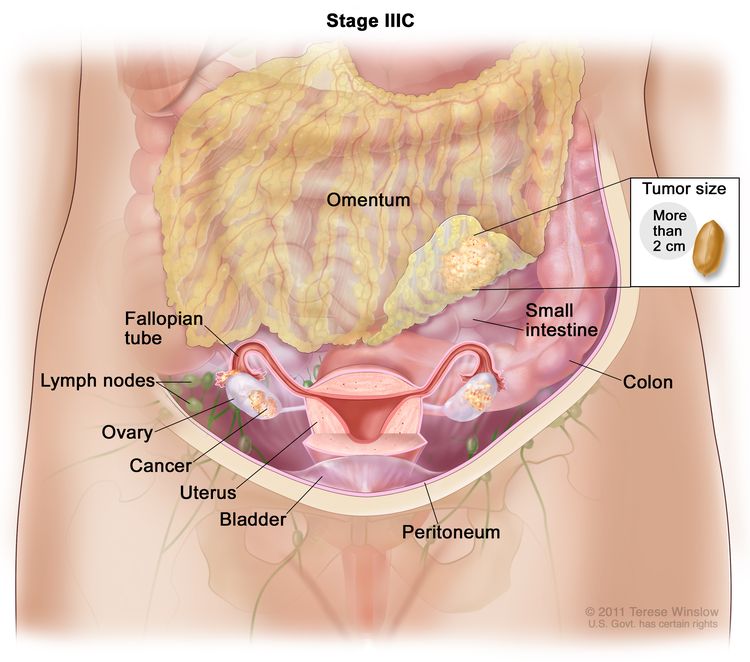
| IIIB | T1–3, N1, M0 | T1 = Tumor invades the lamina propria or muscular layer. |
| –T1a = Tumor invades the lamina propria. | ||
| –T1b = Tumor invades the muscular layer. | ||
| T2 = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). Or, tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. | ||
| –T2a = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). | ||
| –T2b = Tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. | ||
| T3 = Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts. | ||
| N1 = Metastases to one to three regional lymph nodes. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
| IVA | T4, N0–1, M0 | T4 = Tumor invades the main portal vein or hepatic artery or invades two or more extrahepatic organs or structures. |
| N0 = No regional lymph node metastasis. | ||
| N1 = Metastases to one to three regional lymph nodes. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 303–9. | ||
| IVB | Any T, N2, M0 | TX = Primary tumor cannot be assessed. |
| T0 = No evidence of primary tumor. | ||
| Tis = Carcinoma in situ. | ||
| T1 = Tumor invades the lamina propria or muscular layer. | ||
| –T1a = Tumor invades the lamina propria. | ||
| –T1b = Tumor invades the muscular layer. | ||
| T2 = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). Or, tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. | ||
| –T2a = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). | ||
| –T2b = Tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. | ||
| T3 = Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts. | ||
| T4 = Tumor invades the main portal vein or hepatic artery or invades two or more extrahepatic organs or structures. | ||
| N2 = Metastases to four or more regional lymph nodes. | ||
| M0 = No distant metastasis. | ||
| Any T, Any N, M1 | TX = Primary tumor cannot be assessed. | |
| T0 = No evidence of primary tumor. | ||
| Tis = Carcinoma in situ. | ||
| T1 = Tumor invades the lamina propria or muscular layer. | ||
| –T1a = Tumor invades the lamina propria. | ||
| –T1b = Tumor invades the muscular layer. | ||
| T2 = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). Or, tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. | ||
| –T2a = Tumor invades the perimuscular connective tissue on the peritoneal side, without involvement of the serosa (visceral peritoneum). | ||
| –T2b = Tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver. | ||
| T3 = Tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts. | ||
| T4 = Tumor invades the main portal vein or hepatic artery or invades two or more extrahepatic organs or structures. | ||
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node metastasis. | ||
| N1 = Metastases to one to three regional lymph nodes. | ||
| N2 = Metastases to four or more regional lymph nodes. | ||
| M1 = Distant metastases. | ||
Localized and Locally Advanced
Patients with stage I disease have cancer confined to the gallbladder wall that can be completely resected. Patients with stage I tumors that are discovered incidentally and resected during routine cholecystectomy have 5-year survival rates of nearly 100%.[2]
Patients with stage II or III disease have tumors with direct extension into the muscular layer, serosa, or adjacent organs, with or without involvement of locoregional lymph nodes.
Unresectable
Patients with disease that has spread beyond the locoregional lymph nodes or to distant organs have unresectable tumors, and standard therapy is directed at palliation. Patients with earlier-stage disease with poor performance status and/or significant comorbidities may be deemed poor surgical candidates.
References
- Gallbladder. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 303–9.
- Shirai Y, Yoshida K, Tsukada K, et al.: Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg 215 (4): 326-31, 1992. [PUBMED Abstract]
Treatment of Localized and Locally Advanced Gallbladder Cancer
Treatment Options for Localized and Locally Advanced Gallbladder Cancer
Previously unsuspected gallbladder cancer that is incidentally discovered in the mucosa of the gallbladder during pathological examination is curable in more than 80% of patients. However, symptomatic gallbladder cancer that is suspected prior to surgery often penetrates the muscularis and serosa. This type of gallbladder cancer is curable in less than 5% of patients.
One study reported the patterns of lymph node spread from gallbladder cancer and outcomes of patients with metastases to lymph nodes in 111 consecutive surgical patients in a single institution from 1981 to 1995.[1][Level of evidence C1] The standard surgical procedure was removal of the gallbladder, a wedge resection of the liver, resection of the extrahepatic bile duct, and resection of the regional (N1 and N2) lymph nodes. Kaplan-Meier estimates of the 5-year survival rates were 42.5% ± 6.5% for patients with node-negative tumors pathologically staged as T2 to T4 and 31% ± 6.2% for patients with similar node-positive tumors.
Treatment options for localized and locally advanced gallbladder cancer include:
- Surgery.
- External-beam radiation therapy (EBRT).
- Clinical trials exploring the use of radiation therapy and radiosensitizer drugs to improve local control.
Surgery
In patients with previously unsuspected gallbladder cancer that is discovered in the surgical specimen after a routine gallbladder operation and confined to mucosa (T1), most disease is cured.[2,3] During laparoscopic removal of an unsuspected cancer, implantation of carcinoma at all port sites (including the camera site) is possible.[4] All port sites are typically excised completely, even for stage I cancers.
The need for reexploration for more extended resection in incidentally discovered T1b disease is controversial. A multicenter retrospective review identified lymph node metastases in 12% of patients who underwent re-resection after cholecystectomy, but there are no prospective data regarding relative outcome with a second surgery in these patients.[5][Level of evidence C2]
Patients with T2 or T3 disease have higher rates of unsuspected invasive disease at the time of diagnosis. A multicenter retrospective review was performed in patients who underwent re-resection after carcinoma was discovered incidentally. Residual disease was present in 57% of patients with T2 disease (including 31% with lymph node involvement and 10% with liver involvement) and in 77% of patients with T3 disease (including 46% with lymph node metastases and 36% with liver involvement).[5] On the basis of these observations, eligible patients may undergo reexploration to resect liver tissue near the gallbladder bed, portal lymph nodes, and lymphatic tissue in the hepatoduodenal ligament. Retrospective analyses suggest that extended re-resection can delay recurrences and potentially improve survival.[6–8][Level of evidence C2]
For patients with locoregional lymph node involvement (at the cystic duct, common bile duct, hepatic artery, and portal vein), long-term disease-free survival can occasionally be achieved with radical resection. In patients with jaundice (stage III or stage IV), preoperative percutaneous transhepatic biliary drainage for relief of biliary obstruction should be considered.
Surgery with curative intent is not considered possible in patients with metastatic spread beyond the locoregional lymph nodes or to distant organs.
EBRT
The use of EBRT with or without chemotherapy as a primary treatment has been reported to produce short-term disease control in small groups of patients. Similar benefits have been reported for radiation therapy, with or without chemotherapy, administered after resection.[9,10]
There are no phase III studies to support the use of adjuvant radiation therapy, even for patients with high-risk localized disease.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Tsukada K, Kurosaki I, Uchida K, et al.: Lymph node spread from carcinoma of the gallbladder. Cancer 80 (4): 661-7, 1997. [PUBMED Abstract]
- Fong Y, Brennan MF, Turnbull A, et al.: Gallbladder cancer discovered during laparoscopic surgery. Potential for iatrogenic tumor dissemination. Arch Surg 128 (9): 1054-6, 1993. [PUBMED Abstract]
- Chijiiwa K, Tanaka M: Carcinoma of the gallbladder: an appraisal of surgical resection. Surgery 115 (6): 751-6, 1994. [PUBMED Abstract]
- Wibbenmeyer LA, Wade TP, Chen RC, et al.: Laparoscopic cholecystectomy can disseminate in situ carcinoma of the gallbladder. J Am Coll Surg 181 (6): 504-10, 1995. [PUBMED Abstract]
- Pawlik TM, Gleisner AL, Vigano L, et al.: Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 11 (11): 1478-86; discussion 1486-7, 2007. [PUBMED Abstract]
- Shirai Y, Yoshida K, Tsukada K, et al.: Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg 215 (4): 326-31, 1992. [PUBMED Abstract]
- Yamaguchi K, Chijiiwa K, Saiki S, et al.: Retrospective analysis of 70 operations for gallbladder carcinoma. Br J Surg 84 (2): 200-4, 1997. [PUBMED Abstract]
- Downing SR, Cadogan KA, Ortega G, et al.: Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg 146 (6): 734-8, 2011. [PUBMED Abstract]
- Smoron GL: Radiation therapy of carcinoma of gallbladder and biliary tract. Cancer 40 (4): 1422-4, 1977. [PUBMED Abstract]
- Hejna M, Pruckmayer M, Raderer M: The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer 34 (7): 977-86, 1998. [PUBMED Abstract]
Treatment of Unresectable, Metastatic, or Recurrent Gallbladder Cancer
Treatment Options for Unresectable, Metastatic, or Recurrent Gallbladder Cancer
Unresectable, metastatic, and recurrent gallbladder cancers are not curable. Symptoms can be significantly improved with relief of biliary obstruction. A few patients have very slow-growing tumors and may live several years. Patients with unresectable, metastatic, or recurrent gallbladder cancer should consider enrolling in clinical trials whenever possible. Information about ongoing clinical trials is available from the NCI website.
Treatment options for unresectable, metastatic, or recurrent gallbladder cancer include:
Percutaneous transhepatic drainage or endoscopically placed stents, or surgical bypass
Relief of biliary obstruction is warranted when symptoms such as pruritus and hepatic dysfunction outweigh other symptoms of the cancer. The preferred approach is percutaneous transhepatic drainage or endoscopically placed stents.[1] Surgical bypass may be appropriate when these approaches are infeasible.
Palliative radiation therapy after biliary drainage may be beneficial. Patients may be candidates for inclusion in clinical trials that explore ways to improve the effects of radiation therapy with various radiosensitizers, such as hyperthermia, radiosensitizer drugs, or cytotoxic chemotherapeutic agents.
Systemic therapy
Systemic therapy is appropriate for selected patients with adequate performance status and intact organ function. Fluoropyrimidines, gemcitabine, platinum agents, and docetaxel have produced transient partial remissions in a few patients. The incidence of gallbladder cancer is low, and clinical trials often enroll patients with all subsites of biliary tract cancers. Therefore, data must be interpreted with caution when applied specifically to patients with gallbladder cancer. In clinical practice, treatment algorithms in advanced disease often follow those with biliary tract cancer, relying on extrapolation from other trials. In this section, only current landmark studies, or those that specifically enrolled patients with gallbladder cancer, have been explicitly summarized. For more information, see Bile Duct Cancer (Cholangiocarcinoma) Treatment.
Capecitabine and fluorouracil dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[2,3] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[2–4] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient’s DPYD genotype and number of functioning DPYD alleles.[5–7] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[8] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[9]
Evidence (systemic therapy):
- In a phase III study, 410 patients with unresectable metastatic, or recurrent biliary tract cancer were randomly assigned to receive up to 6 months of gemcitabine with or without cisplatin.[10]
- With a median follow-up of 8.2 months, the median overall survival (OS) was superior for patients who received combination chemotherapy (11.7 months vs. 8.1 months; hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.52–0.80; P < .001).[10][Level of evidence A1]
- A similar median OS benefit was demonstrated in all subgroups, including 149 patients with gallbladder cancer.
- Grade 3 and 4 toxicities occurred with similar frequency in both study arms, except for increased hematologic toxic effects in patients who received gemcitabine plus cisplatin and increased hepatotoxicity in patients who received single-agent gemcitabine.
- An international, multicenter, phase III study (TOPAZ-1 [NCT03875235]) included 685 patients with previously untreated and unresectable locally advanced, recurrent, or metastatic biliary tract cancer. Patients were randomly assigned to receive either durvalumab or placebo with cisplatin plus gemcitabine for up to eight cycles, followed by durvalumab or placebo maintenance until disease progression or unacceptable toxicity. After a median follow-up of 23.4 months for patients in the durvalumab group, the following was observed:[11,12]
- The primary end point of median OS was 12.9 months in the durvalumab group and 11.3 months in the placebo group (HR, 0.76; 95% CI, 0.64–0.91). In the durvalumab group, the 18-month OS rate was 35.1%, and the 24-month OS was 24.9%. In the placebo group, the 18-month OS rate was 25.5%, and the 24-month OS rate was 10.4%.[11,12][Level of evidence A1]
- There was no significant difference between groups in the number of grade 3 or 4 treatment-related adverse events or the number of events leading to discontinuation of a study medication.
- An international, multicenter, phase III study (KEYNOTE-966 [NCT04003636]) enrolled 1,069 patients with previously untreated unresectable, locally advanced or metastatic biliary tract cancer. Patients were randomly assigned to receive either pembrolizumab or placebo for up to 35 cycles. This was combined with gemcitabine (with no maximum duration) and cisplatin for up to 8 cycles. After a median follow-up of 25.6 months, the following results were observed:[13][Level of evidence A1]
- The median OS was 12.7 months in the pembrolizumab group and 10.9 months in the placebo group (HR, 0.83; 95% CI, 0.72–0.95; one-sided P = .0034).
- There was no difference in the total frequency of treatment-related adverse events between treatment groups, including grade 3 or grade 4 events. Death due to treatment-related adverse events was seen in a total of eight patients (2%) in the pembrolizumab arm and three patients (1%) in the placebo arm.
- A three-arm randomized phase III study of patients with unresectable gallbladder cancer compared best supportive care (n = 27), fluorouracil plus folinic acid (FUFA) weekly for 30 weeks (n = 28), and modified gemcitabine plus oxaliplatin (mGEMOX) for up to six 21-day cycles.[14][Level of evidence A1]
- With a median follow-up of 9 months, the OS was 4.5 months for patients who received best supportive care (95% CI, 0.2−8.8), 4.6 months for patients who received FUFA (95% CI, 3−6.2), and 9.5 months for patients who received mGEMOX (95% CI, 5−14; P = .039).
- The only significant difference in grade 3 or 4 toxicities between the chemotherapy arms was transaminitis, which was more prevalent in the mGEMOX arm (P = .04).
- A phase III noninferiority study (NCT01470443) enrolled 114 patients with metastatic biliary tract cancers, including 30 (26%) with primary gallbladder cancer. Patients were randomly assigned to receive either capecitabine plus oxaliplatin (XELOX) or gemcitabine plus oxaliplatin (GEMOX).[15][Level of evidence B1]
- OS was not significantly different, at 10.4 months (95% CI, 8.0−12.6) for patients in the GEMOX group and 10.6 months (95% CI, 7.3−15.5) for patients in the XELOX group (P = .131).
- The primary end point of 6-month progression-free survival (PFS) rates were 44.6% for the GEMOX group and 46.7% for the XELOX group (95% CI of difference in 6-month PFS rate, -12% to 16%, meeting criteria for noninferiority).
- A predefined subgroup analysis based on the primary site of disease was performed. The analysis did not reveal a difference in objective response rate between the two arms in patients with gallbladder cancer (P = .598).
Pending additional clinical trials, cisplatin plus gemcitabine is considered the reference standard chemotherapy backbone for patients with unresectable, metastatic, or recurrent gallbladder cancer. Extrapolating from the results of the TOPAZ-1 and KEYNOTE-966 trials for biliary tract cancer, addition of a checkpoint inhibitor (durvalumab or pembrolizumab) to first-line therapy has become the standard of care. Potential alternative regimens include gemcitabine plus capecitabine, GEMOX, and XELOX. All patients should consider clinical trials.
All patients with unresectable, metastatic, or recurrent disease who have not already received checkpoint inhibitors should undergo molecular testing for deficient mismatch repair (dMMR) or microsatellite instability (MSI-H). Extrapolating from a subgroup of patients with gastrointestinal and hepatopancreatobiliary tumors in the I-PREDICT (NCT02534675) and KEYNOTE-158 (NCT02628067) studies, patients with either dMMR or MSI-H tumors can be considered for treatment with pembrolizumab.[16,17][Level of evidence C3]
Additional testing for IDH1 variants, FGFR2 gene fusions, and HER2 expression may provide potential targets in clinical trials. In clinical practice, targeted treatments that have been approved by the U.S. Food and Drug Administration as second-line treatments for patients with cholangiocarcinoma could likely be used to treat appropriate patients with gallbladder cancer. Compelling treatment options for these patients are scarce. For more information, see the Targeted therapy section in Bile Duct Cancer (Cholangiocarcinoma) Treatment.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001. [PUBMED Abstract]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
- Valle J, Wasan H, Palmer DH, et al.: Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362 (14): 1273-81, 2010. [PUBMED Abstract]
- Oh DY, He AR, Qin S, et al.: Updated overall survival (OS) from the phase III TOPAZ-1 study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (+ GC) in patients (pts) with advanced biliary tract cancer (BTC). Ann Oncol 33 (Suppl 7): S565-S566, 2022.
- Oh DY, He AR, Bouattour M, et al.: Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol 9 (8): 694-704, 2024. [PUBMED Abstract]
- Kelley RK, Ueno M, Yoo C, et al.: Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401 (10391): 1853-1865, 2023. [PUBMED Abstract]
- Sharma A, Dwary AD, Mohanti BK, et al.: Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 28 (30): 4581-6, 2010. [PUBMED Abstract]
- Kim ST, Kang JH, Lee J, et al.: Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: a multicenter, open-label, randomized, phase III, noninferiority trial. Ann Oncol 30 (5): 788-795, 2019. [PUBMED Abstract]
- Sicklick JK, Kato S, Okamura R, et al.: Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med 25 (5): 744-750, 2019. [PUBMED Abstract]
- Marabelle A, Le DT, Ascierto PA, et al.: Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 38 (1): 1-10, 2020. [PUBMED Abstract]
Latest Updates to This Summary (02/12/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Gallbladder Cancer
Updated statistics with estimated new cases and deaths for 2025 (cited American Cancer Society as reference 1).
Treatment of Unresectable, Metastatic, or Recurrent Gallbladder Cancer
Revised text about the results of the TOPAZ-1 study which included 685 patients with locally advanced, recurrent, or metastatic biliary tract cancer that was unresectable and previously untreated and randomly assigned them to receive either durvalumab or placebo with cisplatin plus gemcitabine followed by durvalumab or placebo maintenance (cited Oh et al. as reference 12).
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of gallbladder cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Gallbladder Cancer Treatment are:
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Leon Pappas, MD, PhD (Massachusetts General Hospital)
- Ari Seifter, MD (Advocate Health Care)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Gallbladder Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: /types/gallbladder/hp/gallbladder-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389371]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.