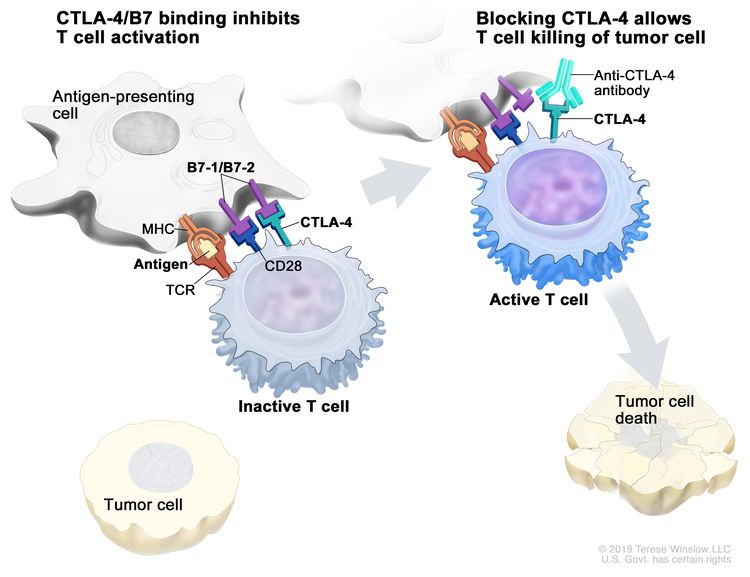
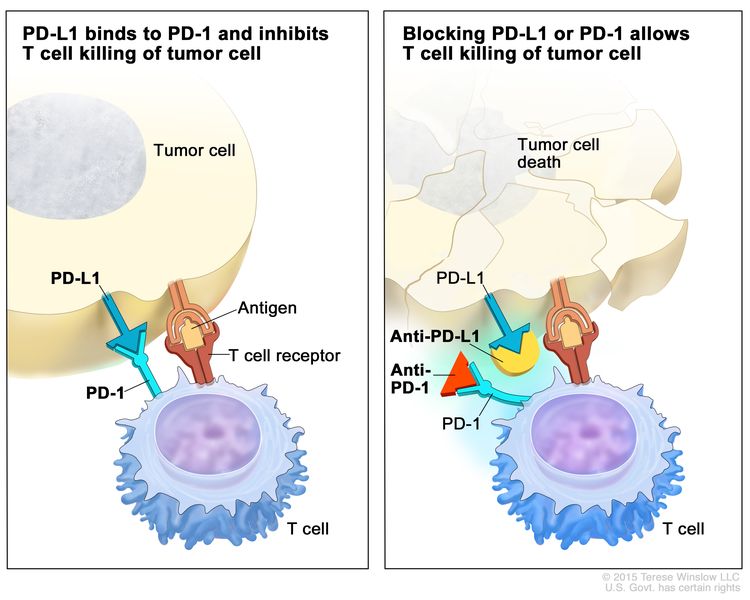
Esophageal Cancer Prevention (PDQ®)–Health Professional Version
Overview
Note: The Overview section summarizes the published evidence on this topic. The rest of the summary describes the evidence in more detail.
Other PDQ summaries on Esophageal Cancer Screening; Esophageal Cancer Treatment; and Levels of Evidence for Cancer Screening and Prevention Studies are also available.
Who Is at Risk?
Smoking and drinking alcohol may account for roughly 90% of esophageal squamous cell carcinoma cases in Western countries like the United States.[1] Gastroesophageal reflux/Barrett esophagus is associated with an increased risk of esophageal adenocarcinoma. Other factors that may explain the increased risk of adenocarcinoma of the esophagus include obesity [2] and the use of medications such as anticholinergics that can predispose to gastroesophageal reflux disease (GERD) by relaxing the lower esophageal sphincter.[3]
Squamous Cell Carcinoma of the Esophagus
Factors with adequate evidence of increased risk of squamous cell carcinoma of the esophagus
Cigarette smoking and drinking alcohol
Based on solid evidence, smoking cigarettes and drinking alcohol increases the risk of esophageal squamous cell carcinoma. Smoking and drinking alcohol may account for roughly 90% of esophageal squamous cell carcinomas in Western countries like the United States.[1]
Magnitude of Effect: Increased risk, moderate magnitude.
- Study Design: Evidence from population-based case-control and cohort studies.
- Internal Validity: Fair.
- Consistency: Good.
- External Validity: Fair.
Factors with adequate evidence of decreased risk of squamous cell carcinoma of the esophagus
Avoidance of tobacco and alcohol
Based on solid evidence, avoidance of tobacco and alcohol would decrease the risk of squamous cell carcinoma.[1,4]
Magnitude of Effect: Large positive benefit.
- Study Design: Evidence obtained from cohort or case-control studies.
- Internal Validity: Fair.
- Consistency: Multiple studies.
- External Validity: Fair.
Chemoprevention
Aspirin and nonsteroidal anti-inflammatory drug (NSAID) use: Benefits
Based on fair evidence, epidemiological studies have found that aspirin or NSAID use is associated with decreased risk of developing or dying of esophageal cancer (odds ratio [OR], 0.57; 95% confidence interval [CI], 0.47–0.71).[5]
Magnitude of Effect: Small positive.
- Study Design: Evidence obtained from cohort or case-control studies.
- Internal Validity: Fair.
- Consistency: Good.
- External Validity: Fair.
Aspirin and NSAID use: Harms
Based on solid evidence, harms of NSAID use include upper gastrointestinal bleeding and serious cardiovascular events, such as myocardial infarction, heart failure, hemorrhagic stroke, and renal impairment.
Magnitude of Effect: Increased risk, small magnitude.
- Study Design: Evidence obtained from randomized controlled trials.
- Internal Validity: Fair.
- Consistency: Good.
- External Validity: Fair.
Adenocarcinoma of the Esophagus
Factors with adequate evidence of increased risk of adenocarcinoma of the esophagus
Gastroesophageal reflux
Based on fair evidence, an association exists between GERD and adenocarcinoma, particularly if the GERD is long-standing and symptoms are severe.[6,7] In a case-control study from Sweden, the OR for patients with recurrent reflux symptoms was 7.7, while the OR for patients with long-standing and severe symptoms was 43.5 (95% CI, 18.3–103.5).[8] A meta-analysis of 1,128 individuals with esophageal adenocarcinoma from five case-control studies reported statistically significant increases in risk with recurrent heartburn (OR, 4.6; 95% CI, 3.3–6.6), regurgitation (OR, 4.6; 95% CI, 3.4–6.1), or both (OR, 4.8; 95% CI, 3.4–6.8). Daily heartburn and regurgitation was associated with an eightfold increase in risk (OR, 8.0; 95% CI, 4.5–14.0).[7]
It is unknown whether elimination of gastroesophageal reflux by surgical or medical means will reduce the risk of adenocarcinoma of the esophagus.[8,9]
Magnitude of Effect: Unknown.
- Study Design: Case-control studies.
- Internal Validity: Fair.
- Consistency: Good; multiple studies.
- External Validity: Fair.
Interventions with adequate evidence of decreased risk of adenocarcinoma of the esophagus
Aspirin and NSAID use: Benefits
Based on fair evidence, epidemiological studies have found that aspirin or NSAID use is associated with decreased risk of developing or dying from esophageal cancer (OR, 0.57; 95% CI, 0.47–0.71).[5,10]
Magnitude of Effect: Unknown magnitude.
- Study Design: Evidence obtained from cohort or case-control studies.
- Internal Validity: Fair.
- Consistency: Good.
- External Validity: Fair.
Aspirin and NSAID use: Harms
Based on solid evidence, harms of NSAID use include upper gastrointestinal bleeding and serious cardiovascular events, such as myocardial infarction, heart failure, hemorrhagic stroke, and renal impairment.
Magnitude of Effect: Increased risk; small magnitude.
- Study Design: Evidence obtained from randomized controlled trials.
- Internal Validity: Good.
- Consistency: Good.
- External Validity: Good.
Ablation of Barrett esophagus with dysplasia: Benefits
A randomized controlled trial has found that radiofrequency ablation of Barrett esophagus with severe dysplasia may lead to eradication of both dysplasia and intestinal metaplasia and a reduced risk of disease progression.[11]
Magnitude of Effect: Impact on cancer mortality not known.
- Study Design: Evidence obtained from a randomized controlled trial.
- Internal Validity: Good.
- Consistency: Single study.
- External Validity: Good.
Ablation of Barrett esophagus with dysplasia: Harms
Based on solid evidence, harms of radiofrequency ablation include esophageal stricture and requirement for dilatation and upper gastrointestinal hemorrhage but at low rates. It is possible that overdiagnosis and overtreatment of Barrett esophagus, particularly without severe dysplasia, could lead to a substantial number of harms.
Magnitude of Effect: The low rates of esophageal stricture and requirement for dilatation and upper gastrointestinal hemorrhage may be an understatement of the risks if this practice is widely adopted by less-experienced physicians.
- Study Design: Evidence obtained from a randomized controlled trial.
- Internal Validity: Good.
- Consistency: Single study.
- External Validity: Patients representative of a subset of people with dysplasia, particularly severe dysplasia; physicians may not be representative of practicing physicians because this is a new technology and requires specialized knowledge.
References
- Engel LS, Chow WH, Vaughan TL, et al.: Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 95 (18): 1404-13, 2003. [PUBMED Abstract]
- Lagergren J: Controversies surrounding body mass, reflux, and risk of oesophageal adenocarcinoma. Lancet Oncol 7 (4): 347-9, 2006. [PUBMED Abstract]
- Lagergren J, Bergström R, Adami HO, et al.: Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med 133 (3): 165-75, 2000. [PUBMED Abstract]
- Siemiatycki J, Krewski D, Franco E, et al.: Associations between cigarette smoking and each of 21 types of cancer: a multi-site case-control study. Int J Epidemiol 24 (3): 504-14, 1995. [PUBMED Abstract]
- Corley DA, Kerlikowske K, Verma R, et al.: Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124 (1): 47-56, 2003. [PUBMED Abstract]
- Lagergren J, Bergström R, Lindgren A, et al.: Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340 (11): 825-31, 1999. [PUBMED Abstract]
- Cook MB, Corley DA, Murray LJ, et al.: Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One 9 (7): e103508, 2014. [PUBMED Abstract]
- Lagergren J, Ye W, Lagergren P, et al.: The risk of esophageal adenocarcinoma after antireflux surgery. Gastroenterology 138 (4): 1297-301, 2010. [PUBMED Abstract]
- Spechler SJ, Goyal RK: The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology 110 (2): 614-21, 1996. [PUBMED Abstract]
- Liao LM, Vaughan TL, Corley DA, et al.: Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 142 (3): 442-452.e5; quiz e22-3, 2012. [PUBMED Abstract]
- Shaheen NJ, Sharma P, Overholt BF, et al.: Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 360 (22): 2277-88, 2009. [PUBMED Abstract]
Background
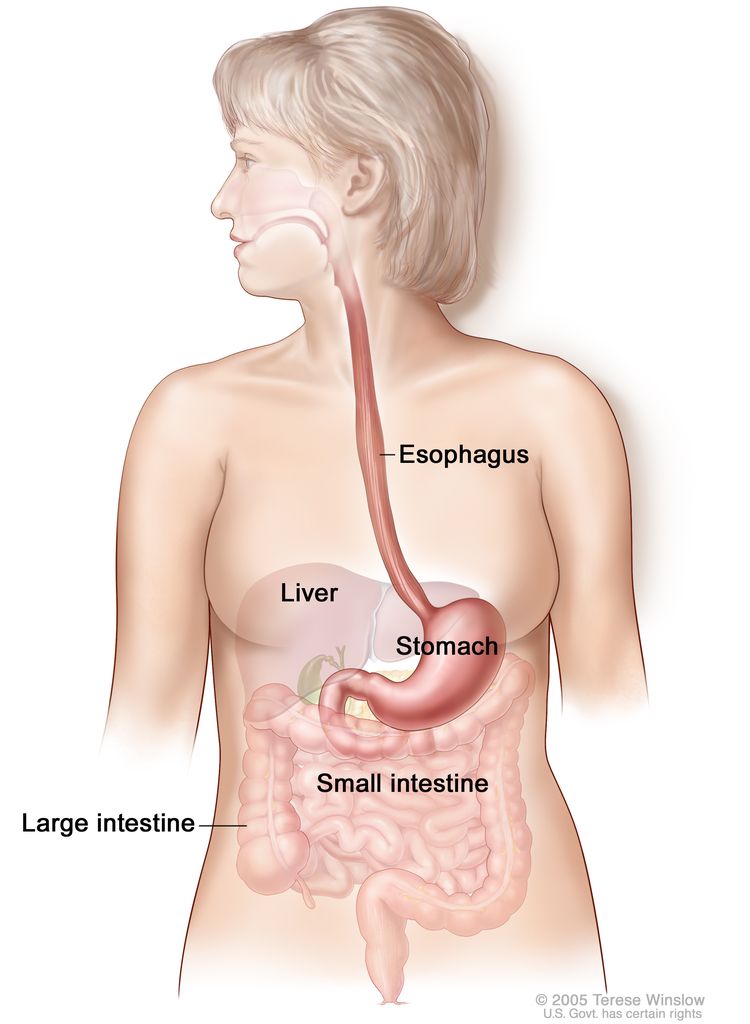
Two histological types account for most malignant esophageal neoplasms: adenocarcinoma and squamous cell carcinoma. The epidemiology of these types varies markedly. In the 1960s, squamous cell carcinomas comprised over 90% of all esophageal tumors. The incidence of esophageal adenocarcinomas has risen markedly for the past two decades. Adenocarcinoma is now more prevalent than squamous cell carcinomas in the United States and Western Europe, with most tumors located in the distal esophagus.[1]
References
- Holmes RS, Vaughan TL: Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol 17 (1): 2-9, 2007. [PUBMED Abstract]
Incidence and Mortality
In 2025, an estimated 22,070 Americans will be diagnosed with esophageal cancer, and 16,250 will die of this disease. Of the new cases, an estimated 17,430 will occur in men, and 4,640 will occur in women.[1] Incidence rates for esophageal cancer have been falling on average 0.4% each year from 2012 to 2021. Death rates have been falling on average 1.1% each year from 2013 to 2022. Incidence rates generally increase with age in all racial and ethnic groups. Incidence rates are higher in White men compared with Black men in all age groups. In women, incidence rates are higher in Black women through age 74 years, at which point the rates become higher in White women.[2] Death rates are higher for White men compared with Black men at all ages. In women, death rates are higher in Black women through age 69 years, at which point the rates become higher in White women.
Although the overall incidence of squamous cell carcinoma of the esophagus is declining, this histological type remains six times more likely to occur in Black men than in White men.[3] In contrast, the incidence of adenocarcinoma of the esophagus rapidly increased from the 1970s to the mid-1990s.[4]
Male sex is an important predictor of adenocarcinoma of the esophagus. The attributable risk is low enough in women that, although the risk from sex is not modifiable, other risk factors necessarily have limited impact.[4]
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed December 30, 2024.
- Devesa SS, Blot WJ, Fraumeni JF: Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 83 (10): 2049-53, 1998. [PUBMED Abstract]
- Hur C, Miller M, Kong CY, et al.: Trends in esophageal adenocarcinoma incidence and mortality. Cancer 119 (6): 1149-58, 2013. [PUBMED Abstract]
Squamous Cell Carcinoma of the Esophagus
Factors With Adequate Evidence of Increased Risk of Squamous Cell Carcinoma of the Esophagus
Smoking cigarettes and drinking alcohol
In the United States, squamous cell carcinoma of the esophagus is strongly associated with tobacco and alcohol abuse. The relative risk associated with tobacco use is 2.4, and the population attributable risk is 54.2% (95% confidence interval [CI], 3.0%–76.2%).[1,2] Retrospective cohort studies adjusted for tobacco use have shown a twofold to sevenfold increase in the risk of esophageal cancer in individuals with alcohol addiction, compared with rates for the general population.[1] Case-control studies have also suggested a significantly increased risk of cancer of the esophagus associated with alcohol abuse.
A multicenter, population-based, case-control study included 221 patients with esophageal squamous cell carcinoma and 695 controls. In this study, ever-smoking, alcohol consumption, and low fruit and vegetable consumption accounted for 56.9% (95% CI, 36.6%–75.1%), 72.4% (95% CI, 53.3%–85.8%), and 28.7% (95% CI, 11.1%–56.5%) of esophageal squamous cell carcinomas, respectively, with a combined population attributable risk of 89.4% (95% CI, 79.1%–95.0%).[3]
In China, where the overall prevalence of esophageal carcinoma is much higher than in the United States, esophageal cancer is associated with deficiencies of nutrients, such as retinol, riboflavin, alpha-carotene, beta-carotene, alpha-tocopherol, ascorbate and zinc, and with exposure to specific carcinogens (e.g., N-nitroso compounds).[1]
Factors With Adequate Evidence of Decreased Risk of Squamous Cell Carcinoma of the Esophagus
Chemoprevention
A prospective, placebo-controlled, esophagus chemoprevention study randomly assigned 610 high-risk Chinese patients.[4] Patients were aged 35 to 64 years and received either placebo or combined low-dose retinol (15 mg or 50,000 IU) plus riboflavin (200 mg) and zinc gluconate (50 mg) for 13.5 months. Standard histological evaluations (including two endoscopic biopsies) were conducted for 93% of all participants. Micronuclei from esophageal cells were obtained before therapy began and after the 13.5 months of treatment. Serum levels of vitamin A, beta-carotene, riboflavin, and zinc were obtained at 0, 2, and 13.5 months.
The second report of this study presented micronuclei frequency results.[5] A statistically significant reduction in the mean percentage of micronucleated esophageal cells occurred in the active-treatment group compared with the placebo group. The pattern of cell proliferation, another potential intermediate end point marker, also improved.[6]
Aspirin and nonsteroidal anti-inflammatory drug (NSAID) use
A systematic review and meta-analysis of the association between aspirin and NSAID use and esophageal cancer identified two cohort and seven case-control studies published between 1980 and 2001.[7] Pooled results showed a protective association between aspirin/NSAID use and esophageal cancer (odds ratio [OR], 0.57; 95% CI, 0.47–0.71). The association with aspirin use was statistically significant (OR, 0.50; 95% CI, 0.38–0.66), and the association with NSAIDs was borderline significant (OR, 0.75; 95% CI, 0.54–1.0). Aspirin/NSAID use was associated with lower risk of both adenocarcinoma (OR, 0.67; 95% CI, 0.51–0.87) and squamous cell carcinoma (OR, 0.58; 95% CI, 0.43–0.78).[7]
References
- Oesophagus. In: World Cancer Research Fund, American Institute for Cancer Research: Food, Nutrition and the Prevention of Cancer: A Global Perspective. The Institute, 1997, pp 118-129.
- Siemiatycki J, Krewski D, Franco E, et al.: Associations between cigarette smoking and each of 21 types of cancer: a multi-site case-control study. Int J Epidemiol 24 (3): 504-14, 1995. [PUBMED Abstract]
- Engel LS, Chow WH, Vaughan TL, et al.: Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 95 (18): 1404-13, 2003. [PUBMED Abstract]
- Muñoz N, Wahrendorf J, Bang LJ, et al.: No effect of riboflavine, retinol, and zinc on prevalence of precancerous lesions of oesophagus. Randomised double-blind intervention study in high-risk population of China. Lancet 2 (8447): 111-4, 1985. [PUBMED Abstract]
- Muñoz N, Hayashi M, Bang LJ, et al.: Effect of riboflavin, retinol, and zinc on micronuclei of buccal mucosa and of esophagus: a randomized double-blind intervention study in China. J Natl Cancer Inst 79 (4): 687-91, 1987. [PUBMED Abstract]
- Yang GC, Lipkin M, Yang K, et al.: Proliferation of esophageal epithelial cells among residents of Linxian, People’s Republic of China. J Natl Cancer Inst 79 (6): 1241-6, 1987. [PUBMED Abstract]
- Corley DA, Kerlikowske K, Verma R, et al.: Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124 (1): 47-56, 2003. [PUBMED Abstract]
Adenocarcinoma of the Esophagus
Factors Associated With Increased Risk of Adenocarcinoma of the Esophagus
Gastroesophageal reflux disease (GERD)
The most important epidemiological difference between squamous cell carcinoma and adenocarcinoma is the strong association between GERD and adenocarcinoma. The results of a population-based case-controlled study suggest that symptomatic gastroesophageal reflux is a risk factor for adenocarcinoma of the esophagus. The frequency, severity, and duration of reflux symptoms were positively associated with an increased risk of adenocarcinoma of the esophagus.[1] In a case-control study from Sweden, the odds ratio (OR) was 7.7 for patients with recurrent reflux symptoms, while the OR for patients with long-standing and severe symptoms was 43.5 (95% confidence interval [CI], 18.3–103.5).[1] A meta-analysis of 1,128 individuals with esophageal adenocarcinoma from five case-control studies reported statistically significant increases in risk with recurrent heartburn (OR, 4.6; 95% CI, 3.3–6.6), regurgitation (OR, 4.6; 95% CI, 3.4–6.1), or both (OR, 4.8; 95% CI, 3.4–6.8). Daily heartburn and regurgitation was associated with an eightfold increase in risk (OR, 8.0; 95% CI, 4.5–14.0).[2] The probable mechanism is that long-standing GERD is associated with the development of Barrett esophagus, a condition in which an abnormal intestinal-type epithelium replaces the stratified squamous epithelium that normally lines the distal esophagus. Barrett esophagus is considered a precursor of esophageal adenocarcinoma.[3] The intestinal-type epithelium of Barrett esophagus has a characteristic endoscopic appearance that differs from squamous epithelium.[4] Dysplasia in Barrett epithelium represents a neoplastic alteration of the columnar epithelium that may progress to invasive adenocarcinoma.[5]
A population-based cohort study in Sweden showed that patients with Barrett esophagus developed adenocarcinoma of the esophagus at about 1.2 cases per 1,000 person-years of follow-up monitoring, which is about 11.3 times higher than in the general population. Thus, while the relative risk may be elevated, the absolute risk is still not high. Furthermore, over half of the cases of adenocarcinoma of the esophagus are not associated with GERD symptoms.
Interventions With Adequate Evidence of Decreased Risk of Adenocarcinoma of the Esophagus
Aspirin and NSAID use
A systematic review and meta-analysis of the association between aspirin and nonsteroidal anti-inflammatory drug (NSAID) use and esophageal cancer identified two cohort and seven case-control studies published between 1980 and 2001.[6] Pooled results showed a protective association between aspirin/NSAID use and esophageal cancer (OR, 0.57; 95% CI, 0.47–0.71). The association with aspirin use was statistically significant (OR, 0.50; 95% CI, 0.38–0.66), and the association with NSAIDs was borderline significant (OR, 0.75; 95% CI, 0.54–1.0). Aspirin/NSAID use was associated with lower risk of both adenocarcinoma (OR, 0.67; 95% CI, 0.51–0.87) and squamous cell carcinoma (OR, 0.58; 95% CI, 0.43–0.78).[6]
Radiofrequency ablation in dysplastic Barrett esophagus
A randomized controlled trial [7] assessed whether radiofrequency ablation (vs. sham ablation) could eradicate dysplastic Barrett esophagus and decrease the rate of neoplastic progression in patients with Barrett esophagus and dysplasia. Among patients with low-grade dysplasia, eradication of dysplasia occurred in 90.5% of the treatment group, compared with 22.7% in the control group. In the high-grade dysplasia group, rates were 81.0% in the treatment group, compared with 19.0% in the control group. Additionally, 77.4% of patients in the ablation group had complete eradication of intestinal metaplasia, compared with 2.3% in the control group. Patients in the ablation group had less disease progression, and although cancer was not a primary outcome because expected numbers were small, there were fewer cancers in the ablation group (1.2% vs. 9.3%; P = .045). The complication rate was relatively low. Among 84 treated patients, there was one upper gastrointestinal hemorrhage and five strictures that were easily treated.[7]
This study suggests that the treatment of patients with Barrett esophagus and dysplasia may ablate Barrett esophagus and prevent disease progression. However, the study provides only weak evidence about whether treatment reduces the outcome of esophageal cancer (because it was not designed to answer that question). Evidence from the study suggests that ablation does not simply coagulate and hide dangerous cells under the surface of the esophagus (those cells could later evolve to cancer). A question entirely separate from this study is whether patients should be screened for Barrett esophagus (this study focused on the treatment of patients with Barrett esophagus who had been identified as having dysplasia). Furthermore, the study does not discuss the net benefits and harms of an overall program of screening (e.g., screening of patients with GERD or certain GERD symptoms) and the surveillance of patients with Barrett esophagus. The potential for overdiagnosis and overtreatment may be considerable if physicians used results of this study to treat patients with Barrett esophagus and no dysplasia.
References
- Lagergren J, Bergström R, Lindgren A, et al.: Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340 (11): 825-31, 1999. [PUBMED Abstract]
- Cook MB, Corley DA, Murray LJ, et al.: Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON). PLoS One 9 (7): e103508, 2014. [PUBMED Abstract]
- Spechler SJ, Goyal RK: The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology 110 (2): 614-21, 1996. [PUBMED Abstract]
- Van Dam J, Brugge WR: Endoscopy of the upper gastrointestinal tract. N Engl J Med 341 (23): 1738-48, 1999. [PUBMED Abstract]
- Reid BJ, Blount PL, Rabinovitch PS: Biomarkers in Barrett’s esophagus. Gastrointest Endosc Clin N Am 13 (2): 369-97, 2003. [PUBMED Abstract]
- Corley DA, Kerlikowske K, Verma R, et al.: Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124 (1): 47-56, 2003. [PUBMED Abstract]
- Shaheen NJ, Sharma P, Overholt BF, et al.: Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 360 (22): 2277-88, 2009. [PUBMED Abstract]
Latest Updates to This Summary (04/10/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Updated statistics with estimated new cases and deaths for 2025 (cited American Cancer Society as reference 1).
This summary is written and maintained by the PDQ Screening and Prevention Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about esophageal cancer prevention. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Screening and Prevention Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Esophageal Cancer Prevention. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: /types/esophageal/hp/esophageal-prevention-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389392]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.