Childhood Extracranial Germ Cell Tumors Treatment (PDQ®)–Health Professional Version
General Information About Childhood Extracranial Germ Cell Tumors (GCTs)
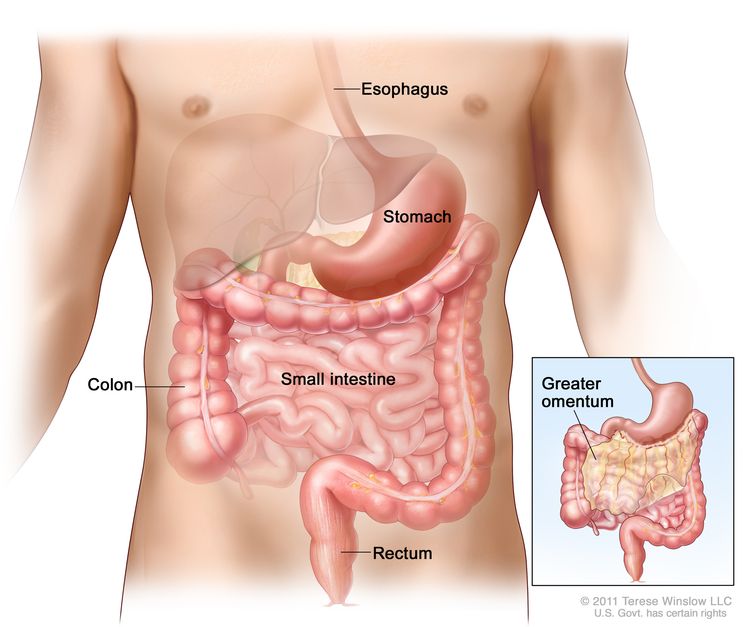
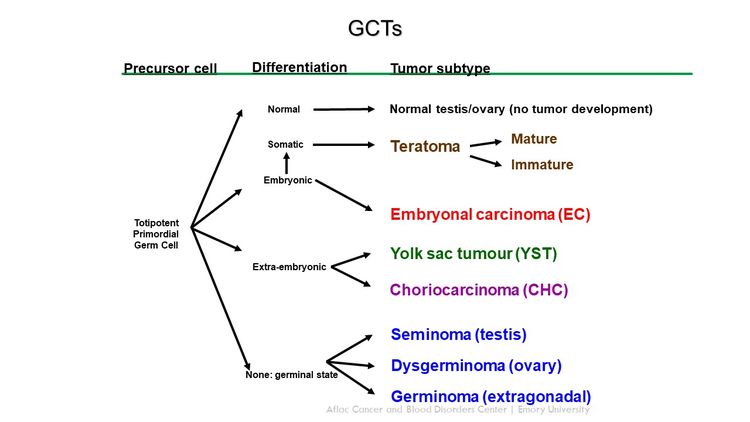
GCTs arise from primordial germ cells, which migrate during embryogenesis from the yolk sac through the mesentery to the gonads (see Figure 1).[1,2] Childhood extracranial GCTs can generally be divided into gonadal and extragonadal. These tumors can also be broadly classified as teratomas, malignant GCTs, or mixed GCTs.
Incidence
Childhood GCTs are rare in children younger than 15 years, accounting for approximately 3% of cancers in this age group.[3–6] In the fetal/neonatal age group, most extracranial GCTs are benign teratomas occurring at midline locations, including the head and neck, sacrococcyx, and retroperitoneum.[7,8] Despite the small percentage of malignant teratomas that occur in this age group, perinatal tumors have a high morbidity rate caused by hydrops fetalis and premature delivery.[8–10]
The incidence of malignant extracranial GCTs increases with the onset of puberty. These tumors represent approximately 15% of cancers in male adolescents aged 15 to 19 years and 4% of cancers in female adolescents aged 15 to 19 years.[3]
Figure 2 shows the age-incidence profile by sex for malignant extracranial/extragonadal GCTs (left panel) and malignant gonadal GCTs (right panel) between 2014 and 2018 for 23 U.S. Cancer registries that represent 66% of all U.S. children, adolescents, and young adults (blue triangles, females; green triangles, males).[3] For males, there is a peak in incidence in children younger than 2 years for both extragonadal and gonadal sites, which is followed by low rates between the ages of 2 and 12 years, and then higher rates throughout adolescence. For females, the peak in young children is present only for extragonadal tumors, with rates increasing after the age of puberty for both extragonadal and gonadal sites. However, the incidence of each tumor is lower for females during adolescence than for males during adolescence.
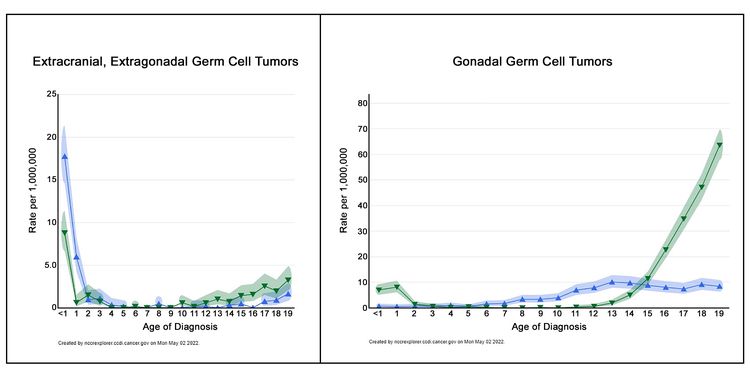
The incidence of extracranial GCTs according to age group, sex, and gonadal versus extragonadal primary site is shown in Table 1.[3]
| Tumor Site | Sex | Age <1 y | Ages 1–4 y | Ages 5–9 y | Ages 10–14 y | Ages 15–19 y |
|---|---|---|---|---|---|---|
| aRates are per 1 million children from 2014 to 2018 for NCCR Registries, 23 U.S. Cancer registries that represent 66% of all U.S. children, adolescents, and young adults. | ||||||
| bData from National Cancer Institute; National Childhood Cancer Registry: NCCR*Explorer.[3] | ||||||
| Extragonadal | Female | 17.7 | 2.1 | 0.1 | 0.1 | 0.7 |
| Male | 8.8 | 0.7 | 0 | 0.6 | 2.2 | |
| Gonadal | Female | 0.6 | 0.7 | 2.1 | 7.6 | 8.3 |
| Male | 7 | 2.5 | 0.1 | 1.5 | 36.1 | |
Risk Factors
Cryptorchidism, the presence of an abdominal undescended testis, has been associated with a 10.8-fold increased risk of developing a GCT.[11] Gonadal dysgenesis, as well as the presence of Y-chromosome material in an abdominal gonad, also increases the risk of developing a gonadal GCT, especially gonadoblastoma. Gonadoblastoma is a rare gonadal tumor consisting of a mixture of germ cells and sex-cord stromal derivatives resembling immature granulosa and Sertoli cells.[12,13]
There are few data about the potential genetic or environmental risk factors associated with childhood extragonadal extracranial GCTs. Patients with the following syndromes are at an increased risk of extragonadal extracranial GCTs:
- Klinefelter syndrome: Increased risk of mediastinal GCTs.[14–17]
Most mediastinal GCTs in adolescents and young adults occur in males, and 22% to 50% have cytogenetic changes consistent with Klinefelter syndrome.[15,18] The age of tumor presentation is younger in patients with Klinefelter syndrome, and testing all younger males for Klinefelter syndrome should be considered.[15,18]
Patients with GCTs were identified from the Children’s Oncology Group (COG) Childhood Cancer Research Network. Twenty-nine patients in the study had mediastinal primary tumors, and nine patients (31%) had Klinefelter syndrome. In the Centers for Disease Control and Prevention’s large 2013 WONDER database, 3% of patients with GCTs had Klinefelter syndrome (70% were mediastinal). In comparison, 0.2% of males in the general population have Klinefelter syndrome.[17]
- Swyer syndrome: Increased risk of gonadoblastomas and seminomas.[19,20]
- Turner syndrome: Increased risk of gonadoblastomas and dysgerminomas.[21,22]
Histological Classification of Childhood Extracranial GCTs
Childhood extracranial GCTs comprise a variety of histological diagnoses and can be broadly classified as the following:
- Teratomas.
- Malignant GCTs.
The histological properties of extracranial GCTs are heterogeneous and vary by primary tumor site and the sex and age of the patient.[23,24] Histologically identical GCTs that arise in younger children have different biological characteristics from those that arise in adolescents and young adults.[25]
Mature teratoma
Mature teratomas can occur at gonadal or at extragonadal locations. They are the most common histological subtype of childhood GCT.[10,26–28] Mature teratomas usually contain well-differentiated tissues from the ectodermal, mesodermal, and endodermal germ cell layers. Any tissue type may be found within this tumor.
Mature teratomas are benign, although some mature teratomas may secrete enzymes or hormones, including insulin, growth hormone, androgens, and prolactin.[29,30]
Immature teratoma
Immature teratomas contain tissues from the ectodermal, mesodermal, and endodermal germ cell layers. Immature tissues, primarily neuroepithelial, are also present. Immature teratomas are graded from 0 to 3 on the basis of the amount of immature neural tissue found in the tumor specimen.[31,32] Tumors of higher grade are more likely to have foci of yolk sac tumor.[33] Immature teratomas can exhibit malignant behavior and metastasize.
Immature teratomas occur primarily in young children at extragonadal sites and in the ovaries of girls near the age of puberty. However, there is no correlation between tumor grade and patient age.[33,34] Some immature teratomas may secrete enzymes or hormones such as vasopressin.[35]
Malignant GCTs
Most childhood extragonadal GCTs arise in midline sites (i.e., head and neck, sacrococcygeal, mediastinal, and retroperitoneal). The midline location may represent aberrant embryonic migration of the primordial germ cells.
GCTs contain malignant tissues of germ cell origin and, rarely, tissues of somatic origin. Isolated malignant elements may constitute a small fraction of a predominantly mature or immature teratoma.[34,36]
Malignant germ cell elements of children, adolescents, and young adults can be grouped broadly by location (see Table 2).
| Malignant Germ Cell Elements | Location | |
|---|---|---|
| E = extragonadal; O = ovarian; T = testicular. | ||
| aModified from Perlman et al.[37] | ||
| Seminomatous | ||
| Seminoma | T | |
| Dysgerminoma | O | |
| Germinoma | E | |
| Nonseminomatous | ||
| Yolk sac tumor (endodermal sinus tumor) | E, O, T | |
| Choriocarcinoma | E, O, T | |
| Embryonal carcinoma | E, T | |
| Gonadoblastoma | O | |
| Mixed Germ Cell Tumors | ||
| Mixed germ cell tumors | E, O, T | |
GCT Biology
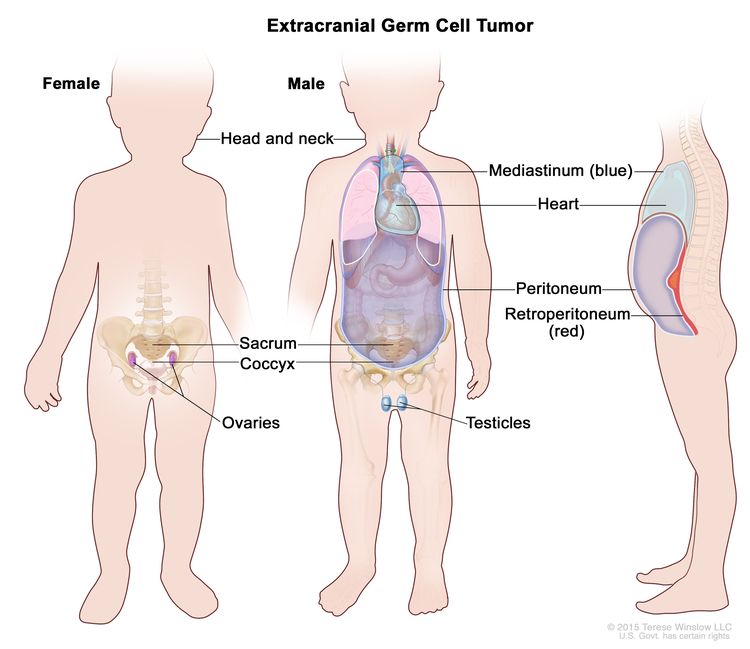
Childhood extracranial GCTs develop at many sites, including testicles, ovaries, mediastinum, retroperitoneum, sacrum, coccyx, and head and neck (see Figure 3).[7] The clinical features at presentation are specific for each site.
The following biologically distinct subtypes of GCTs are found in children and adolescents:
Biological distinctions between GCTs in children and GCTs in adults may not be absolute, and biological factors have not been shown to predict risk.[38–40]
Testicular GCTs
- Children (aged <11 years): During early childhood, both testicular teratomas and malignant testicular GCTs are identified. The malignant tumors are commonly composed of pure yolk sac tumor (also known as endodermal sinus tumor) and are generally diploid or tetraploid. Up to approximately 44% of testicular GCTs contain the isochromosome of the short arm of chromosome 12 (i12p) that characterizes testicular cancer in young adults.[38,41–45] Deletions of chromosomes 1p, 4q, and 6q and gains of chromosomes 1q, 3, and 20q are reported as recurring chromosomal abnormalities for this group of tumors.[43–46]
- Adolescents and young adults (aged ≥11 years): Testicular GCTs in the adolescent and young adult population almost always possess an i12p chromosomal abnormality [47–50] and are aneuploid.[41,50]
Ovarian GCTs
Ovarian GCTs occur primarily in adolescent and young adult females. While most ovarian GCTs are benign mature teratomas (dermoid cysts), a heterogeneous group of malignant GCTs, including immature teratomas, dysgerminomas, yolk sac tumors, and mixed GCTs, do occur in females. The malignant ovarian GCT commonly shows increased copies of the short arm of chromosome 12.[51]
Extragonadal extracranial GCTs
Extragonadal extracranial GCTs occur outside of the brain and gonads.
- Children (aged <11 years): These tumors typically present at birth or during early childhood. Most of these tumors are benign teratomas occurring in the sacrococcygeal region, and thus are not included in Surveillance, Epidemiology, and End Results (SEER) Program data.[52,53] Malignant yolk sac tumor histology occurs in a minority of these tumors; however, they may have cytogenetic abnormalities similar to those observed for tumors occurring in the testes of young males.[42–44,46] Mediastinal GCTs in children younger than 8 years share the same genetic gains and losses as do sacrococcygeal and testicular tumors in young children.[18,54,55]
- Older children, adolescents, and young adults (aged ≥11 years): The mediastinum is the most common primary site for extragonadal GCTs in older children and adolescents.[27]
For information about the treatment of intracranial GCTs, see Childhood Central Nervous System Germ Cell Tumors Treatment.
Diagnostic and Staging Evaluation
Diagnostic evaluation of GCTs includes measurement of serum tumor markers and imaging studies. In suspected cases, tumor markers can suggest the diagnosis before surgery and/or biopsy. This information can be used by the multidisciplinary team to make appropriate treatment choices.
Tumor markers
Tumor markers are measured with each cycle of chemotherapy for all pediatric patients with malignant GCTs. After initial chemotherapy, tumor markers may show a transient elevation.[56]
Common tumor markers include the following:
- Alpha-fetoprotein (AFP).
The fetal liver produces AFP, and during the first year of life, infants have elevated serum AFP levels, which are not associated with the presence of a GCT. Normal ranges have been described.[57,58] The serum half-life of AFP is 5 to 7 days.
Yolk sac tumors produce AFP. Most children with malignant GCTs will have a component of yolk sac tumor and have elevations of AFP levels,[59,60] which are serially monitored during treatment to help assess response to therapy.[34,36,59] Benign teratomas and immature teratomas may produce small elevations of AFP and beta-human chorionic gonadotropin (beta-hCG).
A COG study measured AFP levels in children who received chemotherapy for GCTs. AFP decline was defined as automatically satisfactory if AFP normalized after two cycles of chemotherapy and was calculated satisfactory if the AFP half-life decline was less than or equal to 7 days after the start of chemotherapy. Other decline in AFP was defined as unsatisfactory.[61][Level of evidence C1]
- The cumulative incidence of relapse was 11% for patients with a satisfactory decline in AFP (n = 117) and 38% for patients with an unsatisfactory decline in AFP (n = 14).
- Beta-hCG.
Beta-hCG is produced by all choriocarcinomas and by some germinomas (seminomas and dysgerminomas) and embryonal carcinomas, resulting in elevated serum levels of these substances. The serum half-life of beta-hCG is 1 to 2 days.
- MicroRNAs.
In a prospective multicentric study, the serum level of microRNA-371a-3p was shown to be a sensitive and specific biomarker for adult testicular GCTs.[62] The study included 616 patients with GCTs of varying histologies and 258 controls without malignant GCTs. Elevation of microRNA-371a-3p levels was noted in all malignant histologies, including seminomas. Normal controls and patients with benign teratomas did not have the biomarker elevation. MicroRNA-371a-3p levels were related to tumor volume, and the levels decreased in response to chemotherapy. More studies about microRNA-371a-3p are needed to assess its use in patients with pediatric GCTs.
Imaging tests
Imaging tests may include the following:
- Computed tomography (CT) scan of the chest.
- CT or magnetic resonance imaging (MRI) of the primary site.
- Radionuclide bone scan, if clinically indicated.
- MRI of the brain, if clinically indicated.
Prognostic Factors
Prognostic factors for extracranial GCTs depend on many patient and tumor characteristics and include the following (obtained from historical national GCT trials):[59,63–65]
- Age (e.g., young children vs. adolescents).
- Stage of disease.
- Primary site of disease.
- Histology (e.g., seminomatous vs. nonseminomatous).
- Tumor marker decline (AFP and beta-hCG) in response to therapy.
- Presence of gonadal dysgenesis.
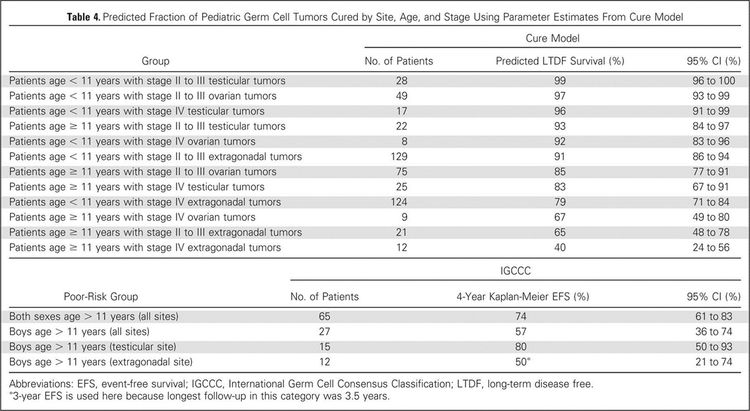
To better identify prognostic factors, data from five U.S. trials and two U.K. trials for malignant extracranial GCTs in children and adolescents were merged by the Malignant Germ Cell Tumor International Collaborative. The goal was to ascertain the important prognostic factors in 519 young patients who received chemotherapy, incorporating age at diagnosis, stage, and site of primary tumor, along with pretreatment AFP level and histology.[66][Level of evidence C2] In this age-focused investigation of these factors in young children and adolescents, outcomes included the following (see Figure 4):[66]
- Patients aged 11 years and older with stage III or stage IV extragonadal disease or stage IV ovarian disease had a less than 70% likelihood of long-term disease-free survival, ranging from 40% (extragonadal stage IV) to 67% (ovarian stage IV).
- Boys (aged 11 years and older) with International Germ Cell Consensus Classification [67] intermediate-risk or poor-risk features also had inferior outcomes.
- Presence of a yolk sac tumor predicted better outcome, but it did not achieve statistical significance at the .05 level.
- Preoperative AFP levels were not prognostic. Postoperative AFP levels were prognostic in adult men.[67]
A subsequent study used a database of 11 GCT trials and identified 593 patients with metastatic testicular, mediastinal, or retroperitoneal GCTs. The distribution of patients by age groups included 90 children (aged 0 to <11 years), 109 adolescents (aged 11 to <18 years), and 394 young adults (aged 18 to ≤30 years).[67]; [68][Level of evidence C1]
- The 5-year event-free survival (EFS) rate was lower for adolescents (72%; 95% confidence interval [CI], 62%–79%) than it was for children (90%; 95% CI, 81%–95%; P = .003) or young adults (88%; 95% CI, 84%–91%; P = .0002).
- After adjusting for the International Germ Cell Consensus Classification risk group,[67] only the difference in EFS between adolescents and children remained significant (hazard ratio, 0.30; P = .001).
Although few pediatric data exist, adult studies have shown that an unsatisfactory decline of elevated tumor markers after the first cycle of chemotherapy is a poor prognostic finding.[69,70]
The presence of gonadal dysgenesis in patients with ovarian nondysgerminomas is associated with worse outcomes. In a report from the COG AGCT0132 study, seven patients with gonadal dysgenesis and ovarian nondysgerminomas had an estimated 3-year EFS rate of 67%, compared with 89% for 100 patients with nondysgerminoma ovarian tumors who did not have gonadal dysgenesis.[13] These dysgenetic gonads contain Y-chromosome material, and intra-abdominal gonads with Y-chromosome material are at increased risk of tumor development.[12,71] In contrast to nondysgerminomas, gonadal dysgenesis was identified in 7 of 48 patients with ovarian dysgerminomas in a report from the French Society of Pediatric Oncology. With a medium follow-up of 14 years, all patients survived.[72]
For more information about prognosis and prognostic factors for childhood extragonadal extracranial GCTs, see the sections on Treatment of Mature and Immature Teratomas in Children, Treatment of Malignant Gonadal GCTs in Children, and Treatment of Malignant Extragonadal Extracranial GCTs in Children.
Follow-up After Treatment
The following tests and procedures may be performed at the physician’s discretion for monitoring children with extracranial GCTs:
- AFP and beta-hCG. Monitor AFP and beta-hCG levels monthly for 6 months (period of highest risk) and then every 3 months, for a total of 2 years (3 years for sacrococcygeal teratoma).
A COG trial of patients with low-risk and intermediate-risk GCTs reported the following results:[73][Level of evidence C2]
- Forty-eight patients with elevated tumor markers at diagnosis relapsed during the surveillance phase.
- At the time of relapse (after central review), 47 of 48 (98%) relapses were detected by tumor marker elevation.
- Imaging tests.
- MRI/CT may be performed at the completion of therapy.
- Guided imaging of the primary site may be performed every 3 months for the first year and every six months for the second year. Seminomas and dysgerminomas may recur later, so the imaging schedule may need to be extended.
- Chest x-ray annually.
- When tumor markers are normal at diagnosis, ultrasonography or CT/MRI may be performed every 3 months for 2 years and then annually for 5 years for germinomas.
Dramatic improvements in survival have been achieved for children and adolescents with cancer.[74] Between 1975 and 2020, childhood cancer mortality decreased by more than 50%.[3,74,75] During the period from 2002 to 2010, cancer mortality continued to decrease by 2.4% per year for children and adolescents with gonadal tumors, as compared with the period from 1975 to 1998 (plateauing from 1998 to 2001).[74] Childhood and adolescent cancer survivors require close monitoring because late effects of cancer therapy may persist or develop months or years after treatment. For information about the incidence, type, and monitoring of late effects of childhood and adolescent cancer survivors, see Late Effects of Treatment for Childhood Cancer.
References
- Dehner LP: Gonadal and extragonadal germ cell neoplasia of childhood. Hum Pathol 14 (6): 493-511, 1983. [PUBMED Abstract]
- McIntyre A, Gilbert D, Goddard N, et al.: Genes, chromosomes and the development of testicular germ cell tumors of adolescents and adults. Genes Chromosomes Cancer 47 (7): 547-57, 2008. [PUBMED Abstract]
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed February 25, 2025.
- Poynter JN, Amatruda JF, Ross JA: Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer 116 (20): 4882-91, 2010. [PUBMED Abstract]
- Kaatsch P, Häfner C, Calaminus G, et al.: Pediatric germ cell tumors from 1987 to 2011: incidence rates, time trends, and survival. Pediatrics 135 (1): e136-43, 2015. [PUBMED Abstract]
- Ward E, DeSantis C, Robbins A, et al.: Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 64 (2): 83-103, 2014 Mar-Apr. [PUBMED Abstract]
- Dharmarajan H, Rouillard-Bazinet N, Chandy BM: Mature and immature pediatric head and neck teratomas: A 15-year review at a large tertiary center. Int J Pediatr Otorhinolaryngol 105: 43-47, 2018. [PUBMED Abstract]
- Isaacs H: Perinatal (fetal and neonatal) germ cell tumors. J Pediatr Surg 39 (7): 1003-13, 2004. [PUBMED Abstract]
- Heerema-McKenney A, Harrison MR, Bratton B, et al.: Congenital teratoma: a clinicopathologic study of 22 fetal and neonatal tumors. Am J Surg Pathol 29 (1): 29-38, 2005. [PUBMED Abstract]
- Alexander VR, Manjaly JG, Pepper CM, et al.: Head and neck teratomas in children–A series of 23 cases at Great Ormond Street Hospital. Int J Pediatr Otorhinolaryngol 79 (12): 2008-14, 2015. [PUBMED Abstract]
- Johnson KJ, Ross JA, Poynter JN, et al.: Paediatric germ cell tumours and congenital abnormalities: a Children’s Oncology Group study. Br J Cancer 101 (3): 518-21, 2009. [PUBMED Abstract]
- Huang H, Wang C, Tian Q: Gonadal tumour risk in 292 phenotypic female patients with disorders of sex development containing Y chromosome or Y-derived sequence. Clin Endocrinol (Oxf) 86 (4): 621-627, 2017. [PUBMED Abstract]
- Dicken BJ, Billmire DF, Krailo M, et al.: Gonadal dysgenesis is associated with worse outcomes in patients with ovarian nondysgerminomatous tumors: A report of the Children’s Oncology Group AGCT 0132 study. Pediatr Blood Cancer 65 (4): , 2018. [PUBMED Abstract]
- Dexeus FH, Logothetis CJ, Chong C, et al.: Genetic abnormalities in men with germ cell tumors. J Urol 140 (1): 80-4, 1988. [PUBMED Abstract]
- Nichols CR, Heerema NA, Palmer C, et al.: Klinefelter’s syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol 5 (8): 1290-4, 1987. [PUBMED Abstract]
- Lachman MF, Kim K, Koo BC: Mediastinal teratoma associated with Klinefelter’s syndrome. Arch Pathol Lab Med 110 (11): 1067-71, 1986. [PUBMED Abstract]
- Williams LA, Pankratz N, Lane J, et al.: Klinefelter syndrome in males with germ cell tumors: A report from the Children’s Oncology Group. Cancer 124 (19): 3900-3908, 2018. [PUBMED Abstract]
- Schneider DT, Schuster AE, Fritsch MK, et al.: Genetic analysis of mediastinal nonseminomatous germ cell tumors in children and adolescents. Genes Chromosomes Cancer 34 (1): 115-25, 2002. [PUBMED Abstract]
- Coutin AS, Hamy A, Fondevilla M, et al.: [Pure 46XY gonadal dysgenesis] J Gynecol Obstet Biol Reprod (Paris) 25 (8): 792-6, 1996. [PUBMED Abstract]
- Amice V, Amice J, Bercovici JP, et al.: Gonadal tumor and H-Y antigen in 46,XY pure gonadal dysgenesis. Cancer 57 (7): 1313-7, 1986. [PUBMED Abstract]
- Tanaka Y, Sasaki Y, Tachibana K, et al.: Gonadal mixed germ cell tumor combined with a large hemangiomatous lesion in a patient with Turner’s syndrome and 45,X/46,X, +mar karyotype. Arch Pathol Lab Med 118 (11): 1135-8, 1994. [PUBMED Abstract]
- Kota SK, Gayatri K, Pani JP, et al.: Dysgerminoma in a female with turner syndrome and Y chromosome material: A case-based review of literature. Indian J Endocrinol Metab 16 (3): 436-40, 2012. [PUBMED Abstract]
- Hawkins EP: Germ cell tumors. Am J Clin Pathol 109 (4 Suppl 1): S82-8, 1998. [PUBMED Abstract]
- Schneider DT, Calaminus G, Koch S, et al.: Epidemiologic analysis of 1,442 children and adolescents registered in the German germ cell tumor protocols. Pediatr Blood Cancer 42 (2): 169-75, 2004. [PUBMED Abstract]
- Horton Z, Schlatter M, Schultz S: Pediatric germ cell tumors. Surg Oncol 16 (3): 205-13, 2007. [PUBMED Abstract]
- Göbel U, Calaminus G, Engert J, et al.: Teratomas in infancy and childhood. Med Pediatr Oncol 31 (1): 8-15, 1998. [PUBMED Abstract]
- Rescorla FJ: Pediatric germ cell tumors. Semin Surg Oncol 16 (2): 144-58, 1999. [PUBMED Abstract]
- Harms D, Zahn S, Göbel U, et al.: Pathology and molecular biology of teratomas in childhood and adolescence. Klin Padiatr 218 (6): 296-302, 2006 Nov-Dec. [PUBMED Abstract]
- Tomlinson MW, Alaverdian AA, Alaverdian V: Testosterone-producing benign cystic teratoma with virilism. A case report. J Reprod Med 41 (12): 924-6, 1996. [PUBMED Abstract]
- Kallis P, Treasure T, Holmes SJ, et al.: Exocrine pancreatic function in mediastinal teratomata: an aid to preoperative diagnosis? Ann Thorac Surg 54 (4): 741-3, 1992. [PUBMED Abstract]
- Norris HJ, Zirkin HJ, Benson WL: Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer 37 (5): 2359-72, 1976. [PUBMED Abstract]
- O’Connor DM, Norris HJ: The influence of grade on the outcome of stage I ovarian immature (malignant) teratomas and the reproducibility of grading. Int J Gynecol Pathol 13 (4): 283-9, 1994. [PUBMED Abstract]
- Heifetz SA, Cushing B, Giller R, et al.: Immature teratomas in children: pathologic considerations: a report from the combined Pediatric Oncology Group/Children’s Cancer Group. Am J Surg Pathol 22 (9): 1115-24, 1998. [PUBMED Abstract]
- Marina NM, Cushing B, Giller R, et al.: Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. J Clin Oncol 17 (7): 2137-43, 1999. [PUBMED Abstract]
- Lam SK, Cheung LP: Inappropriate ADH secretion due to immature ovarian teratoma. Aust N Z J Obstet Gynaecol 36 (1): 104-5, 1996. [PUBMED Abstract]
- Göbel U, Calaminus G, Schneider DT, et al.: The malignant potential of teratomas in infancy and childhood: the MAKEI experiences in non-testicular teratoma and implications for a new protocol. Klin Padiatr 218 (6): 309-14, 2006 Nov-Dec. [PUBMED Abstract]
- Perlman EJ, Hawkins EP: Pediatric germ cell tumors: protocol update for pathologists. Pediatr Dev Pathol 1 (4): 328-35, 1998 Jul-Aug. [PUBMED Abstract]
- Palmer RD, Foster NA, Vowler SL, et al.: Malignant germ cell tumours of childhood: new associations of genomic imbalance. Br J Cancer 96 (4): 667-76, 2007. [PUBMED Abstract]
- Palmer RD, Barbosa-Morais NL, Gooding EL, et al.: Pediatric malignant germ cell tumors show characteristic transcriptome profiles. Cancer Res 68 (11): 4239-47, 2008. [PUBMED Abstract]
- Poynter JN, Hooten AJ, Frazier AL, et al.: Associations between variants in KITLG, SPRY4, BAK1, and DMRT1 and pediatric germ cell tumors. Genes Chromosomes Cancer 51 (3): 266-71, 2012. [PUBMED Abstract]
- Oosterhuis JW, Castedo SM, de Jong B, et al.: Ploidy of primary germ cell tumors of the testis. Pathogenetic and clinical relevance. Lab Invest 60 (1): 14-21, 1989. [PUBMED Abstract]
- Silver SA, Wiley JM, Perlman EJ: DNA ploidy analysis of pediatric germ cell tumors. Mod Pathol 7 (9): 951-6, 1994. [PUBMED Abstract]
- Perlman EJ, Cushing B, Hawkins E, et al.: Cytogenetic analysis of childhood endodermal sinus tumors: a Pediatric Oncology Group study. Pediatr Pathol 14 (4): 695-708, 1994 Jul-Aug. [PUBMED Abstract]
- Schneider DT, Schuster AE, Fritsch MK, et al.: Genetic analysis of childhood germ cell tumors with comparative genomic hybridization. Klin Padiatr 213 (4): 204-11, 2001 Jul-Aug. [PUBMED Abstract]
- Bussey KJ, Lawce HJ, Olson SB, et al.: Chromosome abnormalities of eighty-one pediatric germ cell tumors: sex-, age-, site-, and histopathology-related differences–a Children’s Cancer Group study. Genes Chromosomes Cancer 25 (2): 134-46, 1999. [PUBMED Abstract]
- Perlman EJ, Valentine MB, Griffin CA, et al.: Deletion of 1p36 in childhood endodermal sinus tumors by two-color fluorescence in situ hybridization: a pediatric oncology group study. Genes Chromosomes Cancer 16 (1): 15-20, 1996. [PUBMED Abstract]
- Rodriguez E, Houldsworth J, Reuter VE, et al.: Molecular cytogenetic analysis of i(12p)-negative human male germ cell tumors. Genes Chromosomes Cancer 8 (4): 230-6, 1993. [PUBMED Abstract]
- Bosl GJ, Ilson DH, Rodriguez E, et al.: Clinical relevance of the i(12p) marker chromosome in germ cell tumors. J Natl Cancer Inst 86 (5): 349-55, 1994. [PUBMED Abstract]
- Mostert MC, Verkerk AJ, van de Pol M, et al.: Identification of the critical region of 12p over-representation in testicular germ cell tumors of adolescents and adults. Oncogene 16 (20): 2617-27, 1998. [PUBMED Abstract]
- van Echten J, Oosterhuis JW, Looijenga LH, et al.: No recurrent structural abnormalities apart from i(12p) in primary germ cell tumors of the adult testis. Genes Chromosomes Cancer 14 (2): 133-44, 1995. [PUBMED Abstract]
- Riopel MA, Spellerberg A, Griffin CA, et al.: Genetic analysis of ovarian germ cell tumors by comparative genomic hybridization. Cancer Res 58 (14): 3105-10, 1998. [PUBMED Abstract]
- Malogolowkin MH, Mahour GH, Krailo M, et al.: Germ cell tumors in infancy and childhood: a 45-year experience. Pediatr Pathol 10 (1-2): 231-41, 1990. [PUBMED Abstract]
- Marsden HB, Birch JM, Swindell R: Germ cell tumours of childhood: a review of 137 cases. J Clin Pathol 34 (8): 879-83, 1981. [PUBMED Abstract]
- Dal Cin P, Drochmans A, Moerman P, et al.: Isochromosome 12p in mediastinal germ cell tumor. Cancer Genet Cytogenet 42 (2): 243-51, 1989. [PUBMED Abstract]
- Aly MS, Dal Cin P, Jiskoot P, et al.: Competitive in situ hybridization in a mediastinal germ cell tumor. Cancer Genet Cytogenet 73 (1): 53-6, 1994. [PUBMED Abstract]
- Vogelzang NJ, Lange PH, Goldman A, et al.: Acute changes of alpha-fetoprotein and human chorionic gonadotropin during induction chemotherapy of germ cell tumors. Cancer Res 42 (11): 4855-61, 1982. [PUBMED Abstract]
- Wu JT, Book L, Sudar K: Serum alpha fetoprotein (AFP) levels in normal infants. Pediatr Res 15 (1): 50-2, 1981. [PUBMED Abstract]
- Blohm ME, Vesterling-Hörner D, Calaminus G, et al.: Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol 15 (2): 135-42, 1998 Mar-Apr. [PUBMED Abstract]
- Mann JR, Raafat F, Robinson K, et al.: The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 18 (22): 3809-18, 2000. [PUBMED Abstract]
- Marina N, Fontanesi J, Kun L, et al.: Treatment of childhood germ cell tumors. Review of the St. Jude experience from 1979 to 1988. Cancer 70 (10): 2568-75, 1992. [PUBMED Abstract]
- O’Neill AF, Xia C, Krailo MD, et al.: α-Fetoprotein as a predictor of outcome for children with germ cell tumors: A report from the Malignant Germ Cell International Consortium. Cancer 125 (20): 3649-3656, 2019. [PUBMED Abstract]
- Dieckmann KP, Radtke A, Geczi L, et al.: Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J Clin Oncol 37 (16): 1412-1423, 2019. [PUBMED Abstract]
- Rogers PC, Olson TA, Cullen JW, et al.: Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study–Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J Clin Oncol 22 (17): 3563-9, 2004. [PUBMED Abstract]
- Cushing B, Giller R, Cullen JW, et al.: Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol 22 (13): 2691-700, 2004. [PUBMED Abstract]
- Göbel U, Schneider DT, Calaminus G, et al.: Multimodal treatment of malignant sacrococcygeal germ cell tumors: a prospective analysis of 66 patients of the German cooperative protocols MAKEI 83/86 and 89. J Clin Oncol 19 (7): 1943-50, 2001. [PUBMED Abstract]
- Frazier AL, Hale JP, Rodriguez-Galindo C, et al.: Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 33 (2): 195-201, 2015. [PUBMED Abstract]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 15 (2): 594-603, 1997. [PUBMED Abstract]
- Shaikh F, Stark D, Fonseca A, et al.: Outcomes of adolescent males with extracranial metastatic germ cell tumors: A report from the Malignant Germ Cell Tumor International Consortium. Cancer 127 (2): 193-202, 2021. [PUBMED Abstract]
- Motzer RJ, Nichols CJ, Margolin KA, et al.: Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol 25 (3): 247-56, 2007. [PUBMED Abstract]
- Fizazi K, Pagliaro L, Laplanche A, et al.: Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol 15 (13): 1442-50, 2014. [PUBMED Abstract]
- Thorup J, McLachlan R, Cortes D, et al.: What is new in cryptorchidism and hypospadias–a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg 45 (10): 2074-86, 2010. [PUBMED Abstract]
- Duhil de Bénazé G, Pacquement H, Faure-Conter C, et al.: Paediatric dysgerminoma: Results of three consecutive French germ cell tumours clinical studies (TGM-85/90/95) with late effects study. Eur J Cancer 91: 30-37, 2018. [PUBMED Abstract]
- Fonseca A, Xia C, Lorenzo AJ, et al.: Detection of Relapse by Tumor Markers Versus Imaging in Children and Adolescents With Nongerminomatous Malignant Germ Cell Tumors: A Report From the Children’s Oncology Group. J Clin Oncol 37 (5): 396-402, 2019. [PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed December 30, 2024.
Stage Information for Childhood Extracranial GCTs
As with other childhood solid tumors, stage of disease at diagnosis directly impacts the outcome of patients with malignant germ cell tumors (GCTs).[1–3] The most commonly used staging systems in the United States are as follows:[4]
Testicular GCT Staging From COG (Patients Aged <11 Years)
Table 3 describes the testicular GCT staging for males younger than 11 years from the COG AGCT1531 (NCT03067181) trial.
| Stage | Extent of Disease |
|---|---|
| COG = Children’s Oncology Group; CT = computed tomography; GCT = germ cell tumor. | |
| aMales younger than 50 years are eligible for the AGCT1531 trial. | |
| bCOG trials include patients younger than 15 years with testicular GCT. Although data are scarce, patients between the ages of 11 years and 15 years might be more appropriately staged according to adult testicular guidelines. For more information about the staging of adult testicular GCTs, see Testicular Cancer Treatment. | |
| I | (1) Tumor limited to testis (testes) with negative microscopic margins, completely resected by high inguinal orchiectomy; |
| (2) Tumor capsule cannot have been violated by needle biopsy, incisional biopsy, or tumor rupture. Patients who have undergone scrotal orchiectomy without violation of the tumor capsule and with removal of the spermatic cord to the level of the internal ring are stage I. Patients who have undergone excisional biopsy for frozen section analysis with complete orchiectomy and cord excision at the same operation can be designated stage I; | |
| (3) No clinical, radiographic, or histological evidence of disease beyond the testes; | |
| (4) Lymph nodes all <1 cm maximum short-axis diameter on multiplanar imaging. (Note: Nodes 1–2 cm require short-interval follow-up in 4–6 weeks. If nodes are unchanged at 4–6 weeks [1–2 cm], consider biopsy or transfer to chemotherapy arm. If growing, transfer to chemotherapy arm.) | |
| II | (1) Complete orchiectomy with violation of the tumor capsule in situ (includes preoperative needle biopsy and incisional biopsy or intraoperative tumor capsule rupture); |
| (2) Microscopic disease in scrotum or high in spermatic cord (<5 cm from proximal end). Failure of tumor markers to normalize or decrease with an appropriate half-life; | |
| (3) Lymph nodes negative. | |
| III | (1) Retroperitoneal lymph node involvement, but no visceral or extra-abdominal involvement; |
| (2) Lymph nodes ≥2 cm or lymph nodes >1 cm but <2 cm on short axis by multiplanar imaging CT that fail to resolve on re-imaging at 4–6 weeks. | |
| IV | (1) Distant metastases, including liver, lung, bone, and brain. |
Testicular GCT Staging (Patients Aged ≥11 Years)
Retroperitoneal lymph node dissection has not been required in pediatric germ cell trials to stage disease in males younger than 15 years. Data on adolescent males with testicular GCTs are limited. Retroperitoneal lymph node dissection is used for both staging and treatment in adult testicular GCT trials.[5]
In males older than 15 years, there are only stage I tumors and metastatic tumors. Metastatic tumors are assigned risk according to the International Germ Cell Consensus Classification.[6]
For more information about the American Joint Committee on Cancer staging criteria for testicular GCT in males aged 11 years and older, see Testicular Cancer Treatment.
Ovarian GCT Staging From COG
Table 4 describes the ovarian GCT staging for females younger than 11 years from the COG AGCT1531 (NCT03067181) trial.
| Stage | Extent of Disease | |
|---|---|---|
| COG = Children’s Oncology Group; CT = computed tomography; GCT = germ cell tumor. | ||
| aBilateral ovarian tumors may be any stage as long as other stage criteria are met. Tumor staged according to ovary with most advanced features. | ||
| I | (1) Ovarian tumor removed without violation of the tumor capsule; | |
| (2) No evidence of partial or complete capsular penetration; | ||
| (3) Peritoneal cytology negative for malignant cells; | ||
| (4) Peritoneal surfaces and omentum documented to be free of disease in operative note or biopsied with negative histology if abnormal in appearance; | ||
| (5) Lymph nodes all <1 cm by short-axis diameter on multiplanar imaging or biopsy proven negative. (Note: Nodes 1–2 cm require short-interval follow-up in 4–6 weeks. If nodes are unchanged at 4–6 weeks [1–2 cm], consider biopsy or transfer to chemotherapy arm. If growing, transfer to chemotherapy arm.) | ||
| II | (1) Ovarian tumor completely removed but with preoperative biopsy, violation of tumor capsule in situ, or presence of partial or complete capsule penetration at histology; | |
| (2) Tumor >10 cm removed laparoscopically; | ||
| (3) Tumor morcellated for removal so that capsule cannot be assessed for penetration; | ||
| (4) Peritoneal cytology must be negative for malignant cells; | ||
| (5) Lymph nodes, peritoneal surfaces, and omentum documented to be free of disease in operative note or biopsied with negative histology if abnormal in appearance. | ||
| III | (1) Lymph nodes ≥2 cm or lymph nodes >1 cm but <2 cm on short axis by multiplanar imaging CT that fail to resolve on re-imaging at 4–6 weeks; | |
| (2) Ovarian tumor biopsy or removal with gross residual; | ||
| (3) Positive peritoneal fluid cytology for malignant cells, including immature teratoma; | ||
| (4) Lymph nodes positive for malignant cells, including immature teratoma; | ||
| (5) Peritoneal implants positive for malignant cells, including immature teratoma. | ||
| III–X | Patients otherwise stage I or II by COG criteria but with the following: | |
| (1) Failure to collect peritoneal cytology; | ||
| (2) Failure to biopsy lymph nodes >1 cm on short axis by multiplanar imaging; | ||
| (3) Failure to sample abnormal peritoneal surfaces or omentum; or | ||
| (4) Delayed completion of surgical staging at a second procedure for patients who had only oophorectomy at first procedure. | ||
| IV | (1) Metastatic disease to the parenchyma of the liver (surface implants are stage III) or metastases outside the peritoneal cavity to any other viscera (bone, lung, or brain) and pleural fluid with positive cytology. | |
Ovarian GCT Staging From FIGO
Another ovarian GCT staging system used frequently by gynecologic oncologists is the FIGO staging system, which is based on adequate surgical staging at the time of diagnosis.[7] This system has also been used by some pediatric centers,[2] is most applicable to females older than 11 years, and is described in Table 5. For more information about the FIGO staging system, see Ovarian Germ Cell Tumors Treatment.
| Stage | Description | |
|---|---|---|
| FIGO = International Federation of Gynecology and Obstetrics. | ||
| aAdapted from Berek et al.[8] | ||
| I | Tumor confined to the ovary. | |
| IA | Tumor limited to one ovary (capsule intact); no tumor on surface of the ovary; no malignant cells in the ascites or peritoneal washings. | |
| IB | Tumor limited to both ovaries (capsules intact); no tumor on surface of the ovary; no malignant cells in the ascites or peritoneal washings. | |
| IC | Tumor limited to one or both ovaries, with any of the following: | |
| IC1 | Surgical spill. | |
| IC2 | Capsule ruptured before surgery or tumor on the surface of the ovary. | |
| IC3 | Malignant cells in the ascites or peritoneal washings. | |
| II | Tumor involves one or both ovaries with pelvic extension (below pelvic brim) or primary peritoneal cancer. | |
| IIA | Extension and/or implants on uterus and/or fallopian tubes. | |
| IIB | Extension to other pelvic intraperitoneal tissues. | |
| III | Tumor involves one or both ovaries or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes. | |
| IIIA1 | Positive retroperitoneal lymph nodes only (cytologically or histologically proven): | |
| IIIA1(i) | Lymph nodes ≤10 mm in greatest dimension. | |
| IIIA1(ii) | Lymph nodes >10 mm in greatest dimension. | |
| IIIA2 | Microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes. | |
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis ≤2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes | |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis >2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ). | |
| IV | Distant metastasis excluding peritoneal metastases. | |
| IVA | Pleural effusion with positive cytology. | |
| IVB | Parenchymal metastases and metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity). | |
The ovarian staging systems described above require adherence to specific surgical guidelines. However, in a pediatric intergroup trial, guidelines were followed in only 2 of 131 patients with ovarian tumors.[9] In a single-institution retrospective study, guidelines were followed in only 2 of 44 patients with ovarian tumors.[10]
Extragonadal Extracranial GCT Staging From COG
Table 6 describes the extragonadal extracranial GCT staging from the COG AGCT1531 (NCT03067181) trial.
| Stage | Extent of Disease |
|---|---|
| COG = Children’s Oncology Group; CT = computed tomography; GCT = germ cell tumor. | |
| I | (1) Complete resection at any site, including coccygectomy for sacrococcygeal site; |
| (2) Must have negative tumor margins and intact capsule; | |
| (3) For any tumors involving abdominal cavity or retroperitoneum, peritoneal fluid or washings must be done for cytology and be negative for malignant cells; | |
| (4) Lymph nodes ≤1 cm by imaging of abdomen, pelvis, and chest. (Note: Nodes 1–2 cm require short-interval follow-up in 4–6 weeks. If nodes are unchanged at 4–6 weeks [1–2 cm], consider biopsy or transfer to chemotherapy arm. If growing, transfer to chemotherapy arm. For any tumors involving abdominal cavity or retroperitoneum, peritoneal fluid or washings must be done for cytology and be negative for malignant cells.) | |
| II | (1) Microscopic residual disease; |
| (2) Gross-total resection with preoperative biopsy, intraoperative biopsy, microscopic residual disease, or pathological evidence of capsular disruption; | |
| (3) Lymph nodes negative by abdomen, pelvic, and chest imaging. Peritoneal fluid negative. | |
| III | (1) Gross residual disease or biopsy only; |
| (2) Lymph nodes positive with tumor resection. Lymph nodes ≥2 cm or lymph nodes >1 cm but <2 cm on short axis by multiplanar imaging CT that fail to resolve on re-imaging at 4–6 weeks. | |
| IV | Distant metastases, including liver, lung, bone, and brain. |
References
- Ablin AR, Krailo MD, Ramsay NK, et al.: Results of treatment of malignant germ cell tumors in 93 children: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (10): 1782-92, 1991. [PUBMED Abstract]
- Mann JR, Pearson D, Barrett A, et al.: Results of the United Kingdom Children’s Cancer Study Group’s malignant germ cell tumor studies. Cancer 63 (9): 1657-67, 1989. [PUBMED Abstract]
- Marina N, Fontanesi J, Kun L, et al.: Treatment of childhood germ cell tumors. Review of the St. Jude experience from 1979 to 1988. Cancer 70 (10): 2568-75, 1992. [PUBMED Abstract]
- Brodeur GM, Howarth CB, Pratt CB, et al.: Malignant germ cell tumors in 57 children and adolescents. Cancer 48 (8): 1890-8, 1981. [PUBMED Abstract]
- de Wit R, Fizazi K: Controversies in the management of clinical stage I testis cancer. J Clin Oncol 24 (35): 5482-92, 2006. [PUBMED Abstract]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 15 (2): 594-603, 1997. [PUBMED Abstract]
- Cannistra SA: Cancer of the ovary. N Engl J Med 329 (21): 1550-9, 1993. [PUBMED Abstract]
- Berek JS, Renz M, Kehoe S, et al.: Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 155 (Suppl 1): 61-85, 2021. [PUBMED Abstract]
- Billmire D, Vinocur C, Rescorla F, et al.: Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. J Pediatr Surg 39 (3): 424-9; discussion 424-9, 2004. [PUBMED Abstract]
- Madenci AL, Levine BS, Laufer MR, et al.: Poor adherence to staging guidelines for children with malignant ovarian tumors. J Pediatr Surg 51 (9): 1513-7, 2016. [PUBMED Abstract]
Treatment Option Overview for Childhood Extracranial GCTs
Childhood extracranial germ cell tumors (GCTs) are very heterogenous.
On the basis of clinical factors and tumor histology, appropriate treatment for extracranial GCTs may involve one of the following:
- Surgical resection followed by careful monitoring for disease recurrence.
- Initial surgical resection followed by platinum-based chemotherapy.
- Diagnostic tumor biopsy and preoperative platinum-based chemotherapy followed by definitive tumor resection.[1]
To maximize long-term survival while minimizing treatment-related long-term sequelae (e.g., secondary leukemias, infertility, hearing loss, and renal dysfunction), children with extracranial malignant GCTs need to be cared for at pediatric cancer centers with experience treating these rare tumors.
Treatment Options for Childhood Extracranial GCTs by Histological Type
Table 7 provides an overview of treatment options for children with extracranial GCTs. Specific details of treatment by primary site and clinical condition are described in subsequent sections.
| Histology | Treatment Options | ||
|---|---|---|---|
| BEP = bleomycin (weekly), etoposide, and cisplatin; JEb = carboplatin, etoposide, and bleomycin; PEb = cisplatin, etoposide, and bleomycin (bleomycin only on day 1 of each cycle). | |||
| aChemotherapy has not been shown to be effective in the treatment of children with stages II–IV immature teratoma. However, the role of chemotherapy in these patients has not been systematically studied. In postpubertal patients, chemotherapy remains the standard treatment, although studies are limited.[2] | |||
| bIn prepubertal females with reported stage I disease, but in whom strict surgical staging guidelines were not followed, chemotherapy (PEb) can be considered standard treatment.[3] | |||
| cIn postpubertal females with stage I disease, the strategy of observation after surgery has not been established. This treatment strategy is under investigation in a clinical trial (AGCT1531 [NCT03067181]). | |||
| Mature teratoma | |||
| Sacrococcygeal site | Surgery and observation | ||
| Nonsacrococcygeal site | Surgery and observation | ||
| Immature teratoma | Surgery and observation (stage I) | ||
| Surgery and observation or chemotherapy (stages I–IV) a | |||
| Malignant gonadal GCTs in children: | |||
| Childhood malignant testicular GCTs: | |||
| Malignant testicular GCTs in prepubertal males | Surgery and observation (stage I) | ||
| Surgery and chemotherapy (PEb) (stages II–IV) | |||
| Malignant testicular GCTs in postpubertal males | For information, see Testicular Cancer Treatment. | ||
| Childhood malignant ovarian GCTs: | |||
| Dysgerminomas of the ovary | Surgery and observation (stage I) | ||
| Surgery and chemotherapy (PEb) (stages II–IV) | |||
| Malignant nongerminomatous ovarian GCTs (yolk sac and mixed GCTs) in prepubertal females | Surgery and observation for prepubertal females (stage I following strict surgical staging guidelines) b. For information about the treatment of ovarian mature teratoma, see the Childhood Malignant Ovarian GCTs section. | ||
| Surgery and chemotherapy (PEb) for prepubertal and postpubertal females (purported stage I and stages II–IV) | |||
| Malignant nongerminomatous ovarian GCTs (yolk sac and mixed GCTs) in postpubertal females | Surgery and chemotherapy (BEP) for prepubertal and postpubertal females (purported stage I and stages II–IV) c | ||
| Malignant nongerminomatous ovarian GCTs (yolk sac and mixed GCTs) that are initially unresectable | Biopsy followed by chemotherapy and surgery (initially unresectable ovarian GCT) | ||
| Malignant extragonadal extracranial GCTs in children: | |||
| Malignant extragonadal extracranial GCTs in prepubertal children | Surgery and chemotherapy (PEb or JEb) (stages I –IV) | ||
| Biopsy followed by chemotherapy with or without surgery (stages III and IV) | |||
| Malignant extragonadal extracranial GCTs in postpubertal children | Surgery | ||
| Chemotherapy (BEP) | |||
| Chemotherapy followed by surgery to remove residual tumor | |||
| Enrollment in a clinical trial | |||
| Recurrent malignant GCTs in children | Surgery alone | ||
| Surgery with neoadjuvant or adjuvant chemotherapy | |||
GCTs with non-GCT elements (teratoma with malignant transformation)
The treatment of GCTs with other non-GCT somatic elements is complex, and few data exist to direct treatment. In adolescents, central primitive neuroectodermal tumors and sarcomas have been found in teratomas.[4,5] The Italian Pediatric Germ Cell Tumor group identified 14 patients with malignant GCTs with a somatic malignancy, such as neuroblastoma or rhabdomyosarcoma, embedded in teratomas (<2% of extracranial GCTs).[6]
The optimal treatment strategy for GCTs with non-GCT elements has not been determined. Separate treatments for both malignant GCTs and non-GCT elements may be required.
Surgery
Surgery is an essential component of treatment. Specific treatments will be discussed for each tumor type.
Surgery and Observation
For patients with completely resected immature teratomas of all grades and at any location, and for patients with localized, completely resected (stage I) seminomatous and nonseminomatous GCTs (testicular and ovarian), additional therapy may not be necessary. However, close monitoring of patients is important.[7,8] The watch-and-wait approach requires scheduled serial physical examination, tumor marker determination, and primary tumor imaging to ensure that a recurrent tumor is detected without delay.
Chemotherapy
In the United States, the standard chemotherapy regimen for both adults and children with malignant nonseminomatous GCTs includes cisplatin, etoposide, and bleomycin. Adult patients receive weekly bleomycin throughout treatment (bleomycin, etoposide, and cisplatin [BEP]).[9–12] U.S. pediatric trials included patients aged 15 years and younger with testicular GCTs and patients aged 21 years and younger with ovarian and extragonadal GCTs. Patients received bleomycin only on day 1 of each cycle (cisplatin, etoposide, and bleomycin [PEb]).[3,13] The combination of carboplatin, etoposide, and bleomycin (JEb) underwent clinical investigation in the United Kingdom in children younger than 16 years. Treatment with this regimen produced event-free survival (EFS) rates by site and stage similar to those produced using treatment with PEb.[14,15]; [16][Level of evidence C1] For information about adult BEP and pediatric PEb and JEb chemotherapy dosing schedules, see Table 8.[3,9–11,13] In both adult and pediatric trials, the number of adolescent subjects was small. The optimal therapy for adolescents (aged ≥11 years) is not clear.[17]
The use of JEb appears to be associated with fewer otologic toxic effects and renal toxic effects than does the use of PEb.[14] In a retrospective meta-analysis of data from the Children’s Oncology Group (COG) and the Children’s Cancer and Leukaemia Group germ cell studies conducted contemporaneously, the multivariate cure model showed no difference in 4-year EFS rates. The 4-year EFS rate was 86% (95% confidence interval [CI], 83%–89%) for patients who received the cisplatin regimen (n = 620) and 86% (95% CI, 79%–90%) for patients who received the carboplatin regimen (n = 163) (P = .87).[18][Level of evidence C1] However, PEb and JEb have not been compared in a randomized pediatric GCT trial.
| Regimen | Bleomycin | Etoposide | Cisplatin | Carboplatin |
|---|---|---|---|---|
| BEP = bleomycin, etoposide, and cisplatin; GFR = glomerular filtration rate; JEb = carboplatin, etoposide, and bleomycin; PEb = cisplatin, etoposide, and bleomycin. | ||||
| Adult BEP (every 21 days) [11,19] | 30 units/m2, days 1, 8, 15 (maximum 30 units) | 100 mg/m2, days 1–5 | 20 mg/m2, days 1–5 | |
| Pediatric PEb (every 21 days) [3,13] | 15 units/m2, day 1 (maximum 30 units) | 100 mg/m2, days 1–5 | 20 mg/m2, days 1–5 | |
| Pediatric JEb (every 21–28 days) [14] | 15 units/m2, day 3 (maximum 30 units) | 120 mg/m2, days 1–3 | 600 mg/m2 or GFR-based dosing, day 2 | |
Several trials were conducted by the COG (previously the Children’s Cancer Group and the Pediatric Oncology Group).[3,7,13] These trials explored the use of PEb for the treatment of localized gonadal GCT [3] and intensified regimens for patients with poor-risk features. The strategies included high-dose cisplatin (200 mg/m2) and cyclophosphamide or the protective agent amifostine.[13,20] None of these strategies had a significant effect on survival or decreased toxicity.
The COG conducted a trial of compressed and reduced PEb chemotherapy (three cycles in 3 days) for patients with low-risk or intermediate-risk malignant GCTs. This study was designed as a noninferior trial with a P value of .1. The 4-year EFS rate of 89% was significantly lower than the rate for the historical control model (92%; P = .08).[21] However, the number of patients in each stratum was small, and further investigation in patients with lower-stage disease may be warranted.
Radiation Therapy
Testicular and mediastinal seminomas in males and ovarian dysgerminomas in females are sensitive to radiation, but radiation therapy is rarely recommended because of the known late effects.
References
- Rescorla FJ: Pediatric germ cell tumors. Semin Surg Oncol 16 (2): 144-58, 1999. [PUBMED Abstract]
- Norris HJ, Zirkin HJ, Benson WL: Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer 37 (5): 2359-72, 1976. [PUBMED Abstract]
- Rogers PC, Olson TA, Cullen JW, et al.: Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study–Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J Clin Oncol 22 (17): 3563-9, 2004. [PUBMED Abstract]
- Ehrlich Y, Beck SD, Ulbright TM, et al.: Outcome analysis of patients with transformed teratoma to primitive neuroectodermal tumor. Ann Oncol 21 (9): 1846-50, 2010. [PUBMED Abstract]
- Rice KR, Magers MJ, Beck SD, et al.: Management of germ cell tumors with somatic type malignancy: pathological features, prognostic factors and survival outcomes. J Urol 192 (5): 1403-9, 2014. [PUBMED Abstract]
- Terenziani M, D’Angelo P, Bisogno G, et al.: Teratoma with a malignant somatic component in pediatric patients: the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) experience. Pediatr Blood Cancer 54 (4): 532-7, 2010. [PUBMED Abstract]
- Marina NM, Cushing B, Giller R, et al.: Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. J Clin Oncol 17 (7): 2137-43, 1999. [PUBMED Abstract]
- Schlatter M, Rescorla F, Giller R, et al.: Excellent outcome in patients with stage I germ cell tumors of the testes: a study of the Children’s Cancer Group/Pediatric Oncology Group. J Pediatr Surg 38 (3): 319-24; discussion 319-24, 2003. [PUBMED Abstract]
- de Wit R, Roberts JT, Wilkinson PM, et al.: Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 19 (6): 1629-40, 2001. [PUBMED Abstract]
- Gershenson DM, Morris M, Cangir A, et al.: Treatment of malignant germ cell tumors of the ovary with bleomycin, etoposide, and cisplatin. J Clin Oncol 8 (4): 715-20, 1990. [PUBMED Abstract]
- Williams SD, Birch R, Einhorn LH, et al.: Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med 316 (23): 1435-40, 1987. [PUBMED Abstract]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 15 (2): 594-603, 1997. [PUBMED Abstract]
- Cushing B, Giller R, Cullen JW, et al.: Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol 22 (13): 2691-700, 2004. [PUBMED Abstract]
- Mann JR, Raafat F, Robinson K, et al.: The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 18 (22): 3809-18, 2000. [PUBMED Abstract]
- Stern JW, Bunin N: Prospective study of carboplatin-based chemotherapy for pediatric germ cell tumors. Med Pediatr Oncol 39 (3): 163-7, 2002. [PUBMED Abstract]
- Depani S, Stoneham S, Krailo M, et al.: Results from the UK Children’s Cancer and Leukaemia Group study of extracranial germ cell tumours in children and adolescents (GCIII). Eur J Cancer 118: 49-57, 2019. [PUBMED Abstract]
- Frazier AL, Hale JP, Rodriguez-Galindo C, et al.: Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 33 (2): 195-201, 2015. [PUBMED Abstract]
- Frazier AL, Stoneham S, Rodriguez-Galindo C, et al.: Comparison of carboplatin versus cisplatin in the treatment of paediatric extracranial malignant germ cell tumours: A report of the Malignant Germ Cell International Consortium. Eur J Cancer 98: 30-37, 2018. [PUBMED Abstract]
- Einhorn LH, Williams SD, Loehrer PJ, et al.: Evaluation of optimal duration of chemotherapy in favorable-prognosis disseminated germ cell tumors: a Southeastern Cancer Study Group protocol. J Clin Oncol 7 (3): 387-91, 1989. [PUBMED Abstract]
- Marina N, Chang KW, Malogolowkin M, et al.: Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a Children’s Oncology Group study. Cancer 104 (4): 841-7, 2005. [PUBMED Abstract]
- Shaikh F, Cullen JW, Olson TA, et al.: Reduced and Compressed Cisplatin-Based Chemotherapy in Children and Adolescents With Intermediate-Risk Extracranial Malignant Germ Cell Tumors: A Report From the Children’s Oncology Group. J Clin Oncol 35 (11): 1203-1210, 2017. [PUBMED Abstract]
Special Considerations for the Treatment of Children With Cancer
Cancer in children and adolescents is rare, although the overall incidence has slowly increased since 1975.[1] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following pediatric specialists and others to ensure that children receive treatment, supportive care, and rehabilitation to achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgeons.
- Transplant surgeons.
- Pathologists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists and hematologists.
- Ophthalmologists.
- Rehabilitation specialists.
- Pediatric oncology nurses.
- Social workers.
- Child-life professionals.
- Psychologists.
- Nutritionists.
For specific information about supportive care for children and adolescents with cancer, see the summaries on Supportive and Palliative Care.
The American Academy of Pediatrics has outlined guidelines for pediatric cancer centers and their role in the treatment of children and adolescents with cancer.[2] At these centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate is offered to most patients and their families. Clinical trials for children and adolescents diagnosed with cancer are generally designed to compare potentially better therapy with current standard therapy. Other types of clinical trials test novel therapies when there is no standard therapy for a cancer diagnosis. Most of the progress in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
References
- Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010. [PUBMED Abstract]
- American Academy of Pediatrics: Standards for pediatric cancer centers. Pediatrics 134 (2): 410-4, 2014. Also available online. Last accessed February 25, 2025.
Treatment of Mature and Immature Teratomas in Children
Mature and immature teratomas arise primarily in the sacrococcygeal region of neonates and young children and in the ovaries of pubescent girls. Less commonly, these tumors are found in the testicular region of boys younger than 4 years, the mediastinum of adolescents, and other sites.[1–3] The primary treatment for teratomas is surgery with complete resection. Surgical options for sacrococcygeal teratomas are complex.
Benign head and neck teratomas and immature teratomas can cause morbidity and mortality through obstruction. In preterm infants and neonates, head and neck teratomas and immature teratomas can cause significant airway compromise. In a single-institutional report, airway obstruction was overcome by using the ex utero intrapartum treatment (EXIT) procedure.[4] Complete resection of a teratoma can be achieved.
Treatment of Mature Teratomas
Standard treatment options for mature teratomas (sacrococcygeal sites)
The sacrococcygeal region is the primary tumor site for most benign and malignant germ cell tumors (GCTs) diagnosed in neonates, infants, and children younger than 4 years. These tumors occur more often in girls than in boys; ratios of 3:1 to 4:1 have been reported.[5]
Sacrococcygeal tumors present in the following two clinical patterns related to the child’s age, tumor location, and likelihood of tumor malignancy:[1]
- Neonates: Neonatal tumors present at birth protruding from the sacral site. However, saccrococcygeal tumors may extend into the retroperitoneal space without outward protrusion. They are usually mature or immature teratomas.
- Infants and young children: In infants and young children, tumors present as a palpable mass in the sacro-pelvic region, compressing the bladder or rectum. These pelvic tumors are more likely to be malignant.
The older the child at presentation, the more likely a malignant component is present in addition to the teratoma. An early survey found that the rate of tumor malignancy was 48% for girls and 67% for boys older than 2 months at the time of sacrococcygeal tumor diagnosis, compared with a malignant tumor incidence of 7% for girls and 10% for boys younger than 2 months at the time of diagnosis.[6] The pelvic primary tumor site has been reported to be an adverse prognostic factor. This could be due to a delayed diagnosis because it was overlooked at birth or incomplete resection at the time of original surgery.[6–9]
Standard treatment options for mature teratomas in a sacrococcygeal site include the following:
- Surgery and observation.
Surgery is an essential component of treatment. Complete resection of the coccyx is vital to minimize the likelihood of tumor recurrence.[2]
Standard treatment options for mature teratomas (nonsacrococcygeal sites)
Standard treatment options for mature teratomas in a nonsacrococcygeal site include the following:
- Surgery and observation.
Children with mature teratomas, including mature teratomas of the mediastinum, can be treated with surgery and observation and have an excellent prognosis.[1,10]
In a review of 153 children with nontesticular mature teratomas, the 6-year relapse-free survival rate was 96% for patients with completely resected disease and 55% for patients with incompletely resected disease.[2] Another series included 57 girls with mature teratomas of the ovary. Two patients experienced tumor recurrences (8 and 12 months after ovarian-sparing surgery), and seven patients developed metachronous tumors (as late as 79 months after initial diagnosis).[11][Level of evidence C1]
A multidisciplinary team should treat and monitor neonates with head and neck GCTs. Although most head and neck GCTs are benign, they can be life-threatening and present significant challenges to surgeons, especially in newborns.[4] Some tumors develop malignant elements, which may change the treatment strategy.[12,13]
Mature teratomas in the prepubertal testis are relatively common benign lesions and may be amenable to testis-sparing surgery.[14]
Treatment of Immature Teratomas
Treatment options for immature teratomas
Treatment options for immature teratomas include the following:
- Surgery and observation (stage I).
- Surgery and observation or chemotherapy (stages I–IV). The use of chemotherapy is controversial. For more information, see evidence on the role of chemotherapy for immature teratomas.
Surgery and observation (stage I)
Immature teratomas in children are primarily managed with surgery and observation.
Evidence (surgery and observation for stage I disease):
- A surgery-alone approach was investigated in a study by the Pediatric Oncology Group and Children’s Cancer Group. Surgical resection followed by careful observation was used to treat patients with immature teratomas.[15]
- Surgery alone was curative for most children and adolescents with resected ovarian immature teratomas of any grade, even when elevated levels of serum alpha-fetoprotein (AFP) or microscopic foci of yolk sac tumor were present.
- The 3-year event-free survival (EFS) rates were 97.8% for patients with ovarian tumors, 100% for patients with testicular tumors, and 80% for patients with extragonadal tumors.
Surgery and observation or chemotherapy (stages I–IV)
The use of chemotherapy is controversial in the treatment of immature teratomas. There are no clinical trials supporting the use of chemotherapy in children. In adult women with ovarian tumors, surgery followed by chemotherapy has been the standard treatment approach since 1976.[16] As in children, there are no clinical trials supporting the use of chemotherapy in adults.
Evidence (role of chemotherapy for immature teratomas):
- A seminal article published in 1976 reported that most women with ovarian immature teratomas were treated with surgery and chemotherapy. This approach has remained standard practice in postpubertal females.[16]
- A report on pediatric patients aged 15 years and younger in the United Kingdom found that immature teratomas did not respond to chemotherapy.[17]
- A report from the Malignant Germ Cell Tumor International Collaborative (MaGIC) analyzed data from 98 pediatric patients and 81 adult patients with ovarian immature teratomas. Ninety pediatric patients underwent surgery alone. All 81 adult patients received adjuvant chemotherapy.[18][Level of evidence C1]
- The 5-year EFS rate was 91% for pediatric patients and 98% for adult patients.
- The overall survival (OS) rate was 83% for pediatric patients and 93% for adult patients.
- There were no relapses in patients with grade I tumors. One adult patient with a grade II tumor relapsed after chemotherapy.
- For pediatric patients with grade III, stage I/II tumors, the 5-year EFS rate was 92%. For patients with grade III, stage III tumors, the 5-year EFS rate was 52%.
- For adult patients with grade III, stage I/II tumors, the 5-year EFS rate was 91%. For patients with grade III, stage III/IV tumors, the 5-year EFS rate was 65%.
Additional studies on the treatment of ovarian immature teratomas with chemotherapy are needed. For more information about the treatment of ovarian immature teratomas in postpubertal females, see Ovarian Germ Cell Tumors Treatment.
Treatment options under clinical evaluation for immature teratomas
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- AGCT1531 (NCT03067181) (Active Surveillance, Bleomycin, Carboplatin, Etoposide, or Cisplatin in Treating Pediatric and Adult Patients with GCTs): Patients with ovarian pure-cell immature teratomas that are Children’s Oncology Group stage I (International Federation of Gynecology and Obstetrics [FIGO] stage IA and IB), grade 2 or 3, and have an AFP level of less than 1,000 ng/mL are eligible for surgery and observation on this trial.
Follow-up After Treatment of Mature and Immature Teratomas
After successful resection, neonates diagnosed with benign mature and immature teratomas are closely observed with follow-up exams and serial serum AFP determinations. These tests are done for several years to ensure that AFP measurements normalize to expected physiological levels and to facilitate early detection of tumor relapse.[19,20] Several oncology groups have reported significant rates of recurrence among these benign tumors, ranging from 10% to 21%. Most relapses occur within 3 years of resection.[5,19,21,22]
While there is no standard follow-up schedule, tumor markers are measured frequently for 3 years in all children. With early detection, recurrent malignant GCTs can be treated successfully with surgery and chemotherapy (OS rate, 92%).[23] Long-term survivors are monitored for complications of extensive surgery, which include constipation, fecal and urinary incontinence, and psychologically unacceptable cosmetic scars.[24]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Rescorla FJ: Pediatric germ cell tumors. Semin Surg Oncol 16 (2): 144-58, 1999. [PUBMED Abstract]
- Göbel U, Calaminus G, Engert J, et al.: Teratomas in infancy and childhood. Med Pediatr Oncol 31 (1): 8-15, 1998. [PUBMED Abstract]
- Pinkerton CR: Malignant germ cell tumours in childhood. Eur J Cancer 33 (6): 895-901; discussion 901-2, 1997. [PUBMED Abstract]
- Dharmarajan H, Rouillard-Bazinet N, Chandy BM: Mature and immature pediatric head and neck teratomas: A 15-year review at a large tertiary center. Int J Pediatr Otorhinolaryngol 105: 43-47, 2018. [PUBMED Abstract]
- Rescorla FJ, Sawin RS, Coran AG, et al.: Long-term outcome for infants and children with sacrococcygeal teratoma: a report from the Childrens Cancer Group. J Pediatr Surg 33 (2): 171-6, 1998. [PUBMED Abstract]
- Altman RP, Randolph JG, Lilly JR: Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 9 (3): 389-98, 1974. [PUBMED Abstract]
- Ablin AR, Krailo MD, Ramsay NK, et al.: Results of treatment of malignant germ cell tumors in 93 children: a report from the Childrens Cancer Study Group. J Clin Oncol 9 (10): 1782-92, 1991. [PUBMED Abstract]
- Marina N, Fontanesi J, Kun L, et al.: Treatment of childhood germ cell tumors. Review of the St. Jude experience from 1979 to 1988. Cancer 70 (10): 2568-75, 1992. [PUBMED Abstract]
- Baranzelli MC, Kramar A, Bouffet E, et al.: Prognostic factors in children with localized malignant nonseminomatous germ cell tumors. J Clin Oncol 17 (4): 1212, 1999. [PUBMED Abstract]
- Schneider DT, Calaminus G, Reinhard H, et al.: Primary mediastinal germ cell tumors in children and adolescents: results of the German cooperative protocols MAKEI 83/86, 89, and 96. J Clin Oncol 18 (4): 832-9, 2000. [PUBMED Abstract]
- Braungart S, Craigie RJ, Farrelly P, et al.: Ovarian tumors in children: how common are lesion recurrence and metachronous disease? A UK CCLG Surgeons Cancer Group nationwide study. J Pediatr Surg 55 (10): 2026-2029, 2020. [PUBMED Abstract]
- Bernbeck B, Schneider DT, Bernbeck B, et al.: Germ cell tumors of the head and neck: report from the MAKEI Study Group. Pediatr Blood Cancer 52 (2): 223-6, 2009. [PUBMED Abstract]
- Alexander VR, Manjaly JG, Pepper CM, et al.: Head and neck teratomas in children–A series of 23 cases at Great Ormond Street Hospital. Int J Pediatr Otorhinolaryngol 79 (12): 2008-14, 2015. [PUBMED Abstract]
- Metcalfe PD, Farivar-Mohseni H, Farhat W, et al.: Pediatric testicular tumors: contemporary incidence and efficacy of testicular preserving surgery. J Urol 170 (6 Pt 1): 2412-5; discussion 2415-6, 2003. [PUBMED Abstract]
- Marina NM, Cushing B, Giller R, et al.: Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. J Clin Oncol 17 (7): 2137-43, 1999. [PUBMED Abstract]
- Norris HJ, Zirkin HJ, Benson WL: Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer 37 (5): 2359-72, 1976. [PUBMED Abstract]
- Mann JR, Gray ES, Thornton C, et al.: Mature and immature extracranial teratomas in children: the UK Children’s Cancer Study Group Experience. J Clin Oncol 26 (21): 3590-7, 2008. [PUBMED Abstract]
- Pashankar F, Hale JP, Dang H, et al.: Is adjuvant chemotherapy indicated in ovarian immature teratomas? A combined data analysis from the Malignant Germ Cell Tumor International Collaborative. Cancer 122 (2): 230-7, 2016. [PUBMED Abstract]
- Huddart SN, Mann JR, Robinson K, et al.: Sacrococcygeal teratomas: the UK Children’s Cancer Study Group’s experience. I. Neonatal. Pediatr Surg Int 19 (1-2): 47-51, 2003. [PUBMED Abstract]
- Egler RA, Gosiengfiao Y, Russell H, et al.: Is surgical resection and observation sufficient for stage I and II sacrococcygeal germ cell tumors? A case series and review. Pediatr Blood Cancer 64 (5): , 2017. [PUBMED Abstract]
- Gonzalez-Crussi F, Winkler RF, Mirkin DL: Sacrococcygeal teratomas in infants and children: relationship of histology and prognosis in 40 cases. Arch Pathol Lab Med 102 (8): 420-5, 1978. [PUBMED Abstract]
- Gabra HO, Jesudason EC, McDowell HP, et al.: Sacrococcygeal teratoma–a 25-year experience in a UK regional center. J Pediatr Surg 41 (9): 1513-6, 2006. [PUBMED Abstract]
- De Corti F, Sarnacki S, Patte C, et al.: Prognosis of malignant sacrococcygeal germ cell tumours according to their natural history and surgical management. Surg Oncol 21 (2): e31-7, 2012. [PUBMED Abstract]
- Derikx JP, De Backer A, van de Schoot L, et al.: Long-term functional sequelae of sacrococcygeal teratoma: a national study in The Netherlands. J Pediatr Surg 42 (6): 1122-6, 2007. [PUBMED Abstract]
Treatment of Malignant Gonadal GCTs in Children
Childhood Malignant Testicular GCTs
Malignant testicular GCTs in prepubertal males
The role of surgery at diagnosis for germ cell tumors (GCTs) depends on patient age and tumor site, and treatment must be individualized. All malignant testicular GCTs should be resected. Resection may be followed by subsequent excision of residual masses after chemotherapy.
Testicular GCTs in children occur almost exclusively in boys younger than 5 years.[1,2] The initial surgical approach to evaluate a testicular mass in a young boy is important because a trans-scrotal biopsy can risk inguinal node metastasis.[3,4] Radical inguinal orchiectomy with initial high ligation of the spermatic cord is the procedure of choice.[5]
Computed tomography or magnetic resonance imaging evaluation, with the additional information provided by elevated tumor markers, appears adequate for staging. Retroperitoneal dissection of lymph nodes is not beneficial in the staging of testicular GCTs in young boys.[3,4] Therefore, there may be no reason to risk the potential morbidity (e.g., impotence and retrograde ejaculation) associated with lymph node dissection.[6,7]
A revised risk stratification was developed by the Malignant Germ Cell Tumor International Consortium (see Figure 4).[8]
Standard treatment options for malignant GCTs in prepubertal males
Standard treatment options for malignant GCTs in prepubertal males (aged <11 years) include the following:
The treatment options for malignant GCTs in prepubertal males differ by stage of disease.
Surgery and observation (stage I)
Surgery and close follow-up observation are indicated to document that tumor marker levels normalize after resection.[3,9]
Evidence (surgery and observation for stage I disease in prepubertal males):
- A Children’s Cancer Group (CCG)/Pediatric Oncology Group (POG) clinical trial evaluated surgery followed by observation for boys aged 10 years or younger with stage I testicular tumors.[3,4]
- This treatment strategy resulted in a 6-year event-free survival (EFS) rate of 82%.
- Boys who developed recurrent disease received salvage therapy with four cycles of standard-dose cisplatin, etoposide, and bleomycin (PEb), with a 6-year survival rate of 100%.
- A subsequent Children’s Oncology Group (COG) study of 80 boys younger than 15 years with stage I disease included 15 boys aged 11 to 15 years who were treated with surgery and observation.[10][Level of evidence C1]
- The 4-year EFS rate was 80% for the 65 boys younger than 11 years at diagnosis and 48% for the 15 boys aged 11 years and older (P < .01). All patients’ disease was eventually salvaged, with a 4-year overall survival (OS) rate of 100%.
- Favorable prognostic factors were younger age, presence of pure yolk sac tumor, and lack of lymphovascular invasion by the primary tumor.
- Adult testicular staging systems classify patients with lymphovascular invasion as stage IB. In the entire cohort, those with lymphovascular invasion had a lower 4-year EFS rate (62% vs. 84%).
- A pooled analysis (from the studies above) provided more complete outcome data on patients with stage I disease who were treated with surgery and observation.[11][Level of evidence C2]
- Most patients were prepubertal, but 13.2% of patients were adolescents (aged 13–15 years).
- At a median follow-up of 56 months, all patients were alive.
- There were 25 events.
- On multivariate analysis, independent prognostic factors were age younger than 12 years (hazard ratio [HR], 8.87; P < .0001) and higher pT stage (pT2: HR, 7.31; P = .0017 and pT3: HR, 13.5; P = .0047).
- A German study (MAHO 98) of 128 boys younger than 10 years with testicular GCTs, mostly stage I, also evaluated surgery followed by observation.[12][Level of evidence C1]
- There were 49 patients with yolk sac tumors that were staged as IA after inguinal orchiectomy. Stage IA includes no evidence of lymphovascular invasion. The 5-year EFS rate was 95%, and the 5-year OS rate was 100% for this group. Two patients relapsed and then were cured after chemotherapy.
- There were 12 patients who initially had trans-scrotal orchiectomy who were pathologically confirmed to have no lymphovascular invasion (would be considered stage IA, if not for surgery). Ten patients were observed who had no adverse events. Two patients relapsed (17%) and remained in continuous remission after chemotherapy. No patients had hemiscrotectomy. A long-standing question has been whether trans-scrotal orchiectomy necessitates chemotherapy or hemiscrotectomy.
Surgery and chemotherapy (stages II–IV)
Surgery and chemotherapy with four cycles of standard PEb is a common treatment regimen for prepubertal males with stages II through IV disease. Patients treated with this regimen have OS rates exceeding 90%, suggesting that a reduction in therapy could be considered.[13,14]
Surgery and treatment with four to six cycles of carboplatin, etoposide, and bleomycin (JEb) is an alternative treatment regimen.[9]
Evidence (surgery and chemotherapy for stages II–IV disease in prepubertal males):
- A CCG/POG clinical trial evaluated boys younger than 10 years with stage II tumors who were treated with four cycles of PEb after diagnosis.[13]
- The 6-year EFS and OS rates were 100%.
- In the same CCG/POG clinical trial, boys and adolescents (aged 14 years and younger) with stage III and stage IV testicular tumors were treated with surgical resection followed by four cycles of standard-dose PEb or high-dose PEb (HD-PEb) therapy.[14]
- The 6-year survival rate for males younger than 15 years with stage III and stage IV tumors was 100%.
- The 6-year EFS rate for males younger than 15 years was 100% for stage III tumors and 94% for stage IV tumors.
- The use of HD-PEb therapy did not improve the outcome for these boys but did cause increased incidence of otologic toxic effects.
- European investigators have reported excellent outcomes for boys with testicular GCTs using surgery and observation for stage I tumors and JEb and other cisplatin-containing chemotherapy regimens for stage II, stage III, and stage IV tumors.[6,9]
- A phase III, single-arm COG trial (AGCT0132 [NCT00053352]) included 210 intermediate-risk patients (stages II–IV testicular, stages II–III ovarian, stages I–II extragonadal, or stage I gonadal tumors with subsequent recurrence). These patients received three, rather than four, cycles of PEb and the schedule was compressed from 5 days to 3 days per cycle. A parametric comparator model specified that the observed EFS rate would not be significantly less than 92%.[15][Level of evidence B4]
- The 4-year EFS rate was 89% (95% confidence interval, 83%–92%), which was significantly lower than the 92% threshold of the comparison model (P = .08).
- In a post hoc analysis, the EFS rate was compared with similar patients treated with four cycles of PEb in two previous studies. Among 181 newly diagnosed patients, the 4-year EFS rate was 87%, compared with 92% for 92 comparable children in the historical cohort (P = .15).
- The 4-year EFS rate was significantly associated with stage (stage I, 100%; stage II, 92%; stage III, 85%; and stage IV, 54%; P < .001).
- These data do not support a reduction in the number of cycles of PEb from four to three.
Malignant testicular GCTs in postpubertal males
The treatment options described for prepubertal males may not be strictly applicable to postpubertal males. In particular, retroperitoneal lymph node dissection is a treatment option and may play a crucial role [16] in the initial treatment of patients or in subsequent treatment of patients with residual disease after chemotherapy for metastatic testicular GCT.[17,18] A meta-analysis showed that patients older than 11 years were at higher risk of recurrence.[8] The number of males aged 11 to 15 years with GCT is small; it is possible that these patients should be treated according to adult standards. For more information about the treatment of malignant testicular GCTs in postpubertal males, see Testicular Cancer Treatment.
Standard treatment options for malignant testicular GCTs in postpubertal males
For information about the treatment of malignant testicular GCTs in postpubertal males, see Testicular Cancer Treatment.
Treatment options under clinical evaluation for malignant testicular GCTs
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- AGCT1531 (NCT03067181) (Active Surveillance, Bleomycin, Carboplatin, Etoposide, or Cisplatin in Treating Pediatric and Adult Patients with GCTs): The aim of this trial is to reduce toxicity and maintain efficacy of treatment for patients with standard-risk GCTs. Patients with stage I malignant GCTs (low risk, age 0–50 years) will be treated with surgery and observation. Patients with intermediate-risk GCTs will be randomly assigned to receive cisplatin or carboplatin and bleomycin and etoposide. Children younger than 11 years will receive bleomycin with each cycle, and those aged 11 years and older will receive weekly bleomycin. Patients with pure seminoma or dysgerminoma are excluded from the trial.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Childhood Malignant Ovarian GCTs
Most ovarian neoplasms in children and adolescents are of germ cell origin.[19] Ovarian GCTs are very rare in young girls, but the incidence begins to increase in children aged approximately 8 or 9 years and continues to rise throughout adulthood.[1]
Childhood malignant ovarian GCTs can be divided into germinomatous (dysgerminomas) and nongerminomatous malignant GCTs (i.e., yolk sac carcinomas, mixed GCTs, choriocarcinoma, and embryonal carcinomas).
For more information about childhood mature and immature teratomas arising in the ovary, see the Treatment of Mature Teratomas section. For more information about the treatment of ovarian GCT in postpubertal females, see Ovarian Germ Cell Tumors Treatment.
Dysgerminomas of the ovary
Standard treatment options for dysgerminomas of the ovary
Standard treatment options for dysgerminomas of the ovary include the following:
The treatment options for dysgerminomas of the ovary differ by stage of disease.
Surgery and observation (stage I)
For stage I ovarian dysgerminomas, a cure can usually be achieved by unilateral salpingo-oophorectomy, conserving the uterus and opposite ovary, and close follow-up observation.[9,20–23]
Evidence (surgery and observation for stage I dysgerminomas):
- In three successive French Society of Pediatric Oncology studies (TGM-85, TGM-90, and TGM-95), the following was reported:[24][Level of evidence C1]
- Fifteen patients were identified as stage I, and all patients survived.
- Before 1998, eight patients were treated with adjuvant radiation or chemotherapy. After a practice change in 1998, seven patients underwent surgery and observation.
- One of the seven patients (14%) had a tumor event and responded to treatment with chemotherapy.
Surgery and chemotherapy (stages II–IV)
While advanced-stage ovarian dysgerminomas, like testicular seminomas, are highly curable with surgery and radiation therapy, the effects on growth, fertility, and risk of treatment-induced second malignancy in these young patients [25,26] make chemotherapy a more attractive adjunct to surgery.[27,28] Complete tumor resection is the goal for advanced dysgerminomas. Platinum-based chemotherapy can be given preoperatively to facilitate resection or postoperatively (after debulking surgery) to avoid mutilating surgical procedures.[23]
Evidence (surgery and chemotherapy for stage II–IV dysgerminomas):
- In a report from the French Society of Pediatric Oncology, 48 girls younger than 19 years (median age, 12.8 years) were registered; 20 patients had localized disease, 28 patients had locoregional disease, and no patients had metastases. Seven patients had positive para-aortic lymph nodes. Forty-seven patients underwent primary surgery. Before 1998, all patients with advanced disease received radiation therapy. After a practice change in 1998, patients were treated with platinum-based chemotherapy.[24][Level of evidence C1]
- The 5-year EFS rate was 91%, and the 5-year OS rate was 100%.
- Side effects were not severe, and several patients became pregnant in later years.
- A meta-analysis of patients with dysgerminoma by the Malignant Germ Cell Tumor International Consortium reported the following:[29]
- No difference was seen between patients who were treated with cisplatin (n = 70) (5-year EFS rate, 93%; OS rate, 96%) and patients who were treated with carboplatin (n = 56) (5-year EFS rate, 96%; OS rate, 96%).
This approach results in a high rate of cure and the preservation of menstrual function and fertility in most patients with dysgerminomas.[27,30]
Malignant nongerminomatous ovarian GCTs
A multidisciplinary approach is essential for treatment of ovarian GCTs. Various surgical subspecialists and the pediatric oncologist must be involved in clinical decisions. The surgical approach for pediatric ovarian GCTs is often guided by the expectation that reproductive function can be preserved.
The treatment of ovarian malignant GCTs that are not dysgerminomas or immature teratomas generally involves surgical resection and adjuvant chemotherapy.[31,32]
The role for surgery at diagnosis depends on patient age and tumor site, and treatment must be individualized. The use of laparoscopy in children with ovarian GCTs has not been well studied.
The use of intraoperative frozen biopsy in pediatric and adolescent patients to determine the presence of malignancy to allow an ovarian-sparing procedure has been questioned. In a retrospective analysis from the COG AGCT0132 (NCT00053352) study, 60 of 131 eligible patients with ovarian tumors had both intraoperative frozen section and final paraffin section diagnoses available.[33] Intraoperative frozen section biopsy was incorrect 38% of the time (23 patients), and it confirmed the final diagnosis 76% of the time. In addition, central pathological review detected additional germ cell components in 23.7% of patients.
Pediatric surgical guidelines to determine stage I disease have been published.[34] Adult surgical guidelines to determine stage are more extensive. For more information about staging of ovarian GCTs in postpubertal females, see the Stage Information for Ovarian Germ Cell Tumors section in Ovarian Germ Cell Tumors Treatment.
Strict surgical staging guidelines need to be followed to determine true stage I disease. Historically, in both pediatric and adult studies, comprehensive staging guidelines have not been followed. If strict surgical staging guidelines are not followed, surgery followed by chemotherapy, rather than surgery followed by observation, is the standard treatment.[9,13,35]
A goal of surgical therapy for pediatric GCTs is to preserve reproductive function. If conservative surgery is the choice, a high rate of cure can be obtained with adjuvant chemotherapy, and adherence to strict surgical guidelines is not necessary.[36]
Standard treatment options for malignant nongerminomatous ovarian GCTs
Standard treatment options for malignant nongerminomatous ovarian GCTs in prepubertal females include the following:
Standard treatment options for malignant nongerminomatous ovarian GCTs in postpubertal females include the following:
Standard treatment options for malignant nongerminomatous ovarian GCTs that cannot be resected initially include the following:
Surgery and observation for prepubertal females (stage I following strict surgical staging guidelines)
When strict surgical staging guidelines are followed, surgery followed by observation may be an appropriate treatment choice for prepubertal females with stage I disease.
Evidence (surgery and observation for prepubertal females with stage I disease):
- In a COG trial, 25 girls with stage I ovarian malignant GCTs were treated with surgery and observation.[34]
- The 4-year EFS rate was 52%.
- Relapse was detected in 12 patients by tumor marker elevation (mean time, 2 months). All patients later received salvage therapy with three cycles of PEb. The 4-year OS rate was 96%; one patient’s disease was not salvaged.
- Similar results have been reported in other international pediatric trials, but the number of patients has been small.[9]
Surgery and chemotherapy for prepubertal and postpubertal females (purported stage I and stages II–IV)
A revised risk stratification was developed by the Malignant Germ Cell Tumor International Consortium (see Figure 4).[8]
Chemotherapy regimens with cisplatin (PEb) or carboplatin (JEb) have been used successfully in children.[9,13,14,20] BEP is a common regimen in young women with ovarian GCTs.[37,38] BEP differs from PEb with the addition of weekly bleomycin. This approach results in a high rate of cure and the preservation of menstrual function and fertility in most patients with nondysgerminomas.[32,35] For more information about the dosing schedules for BEP, PEb, and JEb, see Table 8.
In prepubertal females with purported stage I ovarian tumors (when strict surgical staging guidelines are not followed) surgery followed by chemotherapy (four cycles of PEb) is an appropriate treatment choice and results in EFS and OS rates of 95%.[13,14]
In postpubertal females with purported stage I ovarian tumors, chemotherapy after resection remains the standard treatment. In postpubertal females, the strategy of observation after surgery has not been established and is under investigation in the AGCT1531 (NCT03067181) trial.
In prepubertal and postpubertal females with stages II, III, or IV ovarian tumors, surgery and chemotherapy are considered standard treatments. Surgery and chemotherapy with four to six cycles of standard PEb is used to treat younger (prepubertal) girls,[13,14] and BEP is used to treat postpubertal girls.[37,38] Patients with normalization of tumor markers undergo imaging after four cycles of PEb, and any residual tumor is resected. Patients with residual viable tumor after surgery are considered refractory.
Alternatively, surgery and chemotherapy with four to six cycles of JEb is a treatment option (as demonstrated in one study in which all patients were younger than 15 years).[9]
Initially unresectable ovarian GCT
Primary resection of ovarian GCT is usually attempted. However, in rare instances, primary resection of the ovary is not possible without undue risk of damage to adjacent structures. In these cases, an appropriate strategy is biopsy for diagnosis, followed by chemotherapy and then subsequent surgery for patients who have residual masses after undergoing chemotherapy.
Treatment options under clinical evaluation for malignant ovarian GCTs
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- AGCT1531 (NCT03067181) (Active Surveillance, Bleomycin, Carboplatin, Etoposide, or Cisplatin in Treating Pediatric and Adult Patients with GCTs): The aim of this trial is to reduce toxicity and maintain efficacy of treatment for patients with standard-risk GCTs. Patients with stage I malignant GCTs (low risk, age 0–50 years) will be treated with surgery and observation. Patients with intermediate-risk GCTs will be randomly assigned to receive cisplatin or carboplatin and bleomycin and etoposide. Children younger than 11 years will receive bleomycin with each cycle, and those aged 11 years and older will receive weekly bleomycin. Patients with pure seminoma or dysgerminoma are excluded from the trial.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute. Available online. Last accessed February 25, 2025.
- Walsh TJ, Grady RW, Porter MP, et al.: Incidence of testicular germ cell cancers in U.S. children: SEER program experience 1973 to 2000. Urology 68 (2): 402-5; discussion 405, 2006. [PUBMED Abstract]
- Schlatter M, Rescorla F, Giller R, et al.: Excellent outcome in patients with stage I germ cell tumors of the testes: a study of the Children’s Cancer Group/Pediatric Oncology Group. J Pediatr Surg 38 (3): 319-24; discussion 319-24, 2003. [PUBMED Abstract]
- Canning DA: Excellent outcome in patients with stage I germ cell tumors of the testes: a study of the Children’s Cancer Group/Pediatric Oncology Group [Editorial Comment on Schlatter]. J Urol 174 (1): 310, 2005.
- Rescorla FJ: Pediatric germ cell tumors. Semin Surg Oncol 16 (2): 144-58, 1999. [PUBMED Abstract]
- Haas RJ, Schmidt P, Göbel U, et al.: Treatment of malignant testicular tumors in childhood: results of the German National Study 1982-1992. Med Pediatr Oncol 23 (5): 400-5, 1994. [PUBMED Abstract]
- Pinkerton CR: Malignant germ cell tumours in childhood. Eur J Cancer 33 (6): 895-901; discussion 901-2, 1997. [PUBMED Abstract]
- Frazier AL, Hale JP, Rodriguez-Galindo C, et al.: Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 33 (2): 195-201, 2015. [PUBMED Abstract]
- Mann JR, Raafat F, Robinson K, et al.: The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 18 (22): 3809-18, 2000. [PUBMED Abstract]
- Rescorla FJ, Ross JH, Billmire DF, et al.: Surveillance after initial surgery for Stage I pediatric and adolescent boys with malignant testicular germ cell tumors: Report from the Children’s Oncology Group. J Pediatr Surg 50 (6): 1000-3, 2015. [PUBMED Abstract]
- Singla N, Wong J, Singla S, et al.: Clinicopathologic predictors of outcomes in children with stage I testicular germ cell tumors: A pooled post hoc analysis of trials from the Children’s Oncology Group. J Pediatr Urol 18 (4): 505-511, 2022. [PUBMED Abstract]
- Göbel U, Haas R, Calaminus G, et al.: Testicular germ cell tumors in boys <10 years: results of the protocol MAHO 98 in respect to surgery and watch & wait strategy. Klin Padiatr 225 (6): 296-302, 2013. [PUBMED Abstract]
- Rogers PC, Olson TA, Cullen JW, et al.: Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study–Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J Clin Oncol 22 (17): 3563-9, 2004. [PUBMED Abstract]
- Cushing B, Giller R, Cullen JW, et al.: Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol 22 (13): 2691-700, 2004. [PUBMED Abstract]
- Shaikh F, Cullen JW, Olson TA, et al.: Reduced and Compressed Cisplatin-Based Chemotherapy in Children and Adolescents With Intermediate-Risk Extracranial Malignant Germ Cell Tumors: A Report From the Children’s Oncology Group. J Clin Oncol 35 (11): 1203-1210, 2017. [PUBMED Abstract]
- de Wit R, Fizazi K: Controversies in the management of clinical stage I testis cancer. J Clin Oncol 24 (35): 5482-92, 2006. [PUBMED Abstract]
- Carver BS, Shayegan B, Serio A, et al.: Long-term clinical outcome after postchemotherapy retroperitoneal lymph node dissection in men with residual teratoma. J Clin Oncol 25 (9): 1033-7, 2007. [PUBMED Abstract]
- Carver BS, Shayegan B, Eggener S, et al.: Incidence of metastatic nonseminomatous germ cell tumor outside the boundaries of a modified postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol 25 (28): 4365-9, 2007. [PUBMED Abstract]
- Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649. Also available online. Last accessed December 22, 2023.
- Baranzelli MC, Bouffet E, Quintana E, et al.: Non-seminomatous ovarian germ cell tumours in children. Eur J Cancer 36 (3): 376-83, 2000. [PUBMED Abstract]
- Dark GG, Bower M, Newlands ES, et al.: Surveillance policy for stage I ovarian germ cell tumors. J Clin Oncol 15 (2): 620-4, 1997. [PUBMED Abstract]
- Marina NM, Cushing B, Giller R, et al.: Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. J Clin Oncol 17 (7): 2137-43, 1999. [PUBMED Abstract]
- Gershenson DM: Chemotherapy of ovarian germ cell tumors and sex cord stromal tumors. Semin Surg Oncol 10 (4): 290-8, 1994 Jul-Aug. [PUBMED Abstract]
- Duhil de Bénazé G, Pacquement H, Faure-Conter C, et al.: Paediatric dysgerminoma: Results of three consecutive French germ cell tumours clinical studies (TGM-85/90/95) with late effects study. Eur J Cancer 91: 30-37, 2018. [PUBMED Abstract]
- Teinturier C, Gelez J, Flamant F, et al.: Pure dysgerminoma of the ovary in childhood: treatment results and sequelae. Med Pediatr Oncol 23 (1): 1-7, 1994. [PUBMED Abstract]
- Mitchell MF, Gershenson DM, Soeters RP, et al.: The long-term effects of radiation therapy on patients with ovarian dysgerminoma. Cancer 67 (4): 1084-90, 1991. [PUBMED Abstract]
- Brewer M, Gershenson DM, Herzog CE, et al.: Outcome and reproductive function after chemotherapy for ovarian dysgerminoma. J Clin Oncol 17 (9): 2670-75, 1999. [PUBMED Abstract]
- Williams SD, Blessing JA, Hatch KD, et al.: Chemotherapy of advanced dysgerminoma: trials of the Gynecologic Oncology Group. J Clin Oncol 9 (11): 1950-5, 1991. [PUBMED Abstract]
- Shah R, Xia C, Krailo M, et al.: Is carboplatin-based chemotherapy as effective as cisplatin-based chemotherapy in the treatment of advanced-stage dysgerminoma in children, adolescents and young adults? Gynecol Oncol 150 (2): 253-260, 2018. [PUBMED Abstract]
- Gershenson DM: Menstrual and reproductive function after treatment with combination chemotherapy for malignant ovarian germ cell tumors. J Clin Oncol 6 (2): 270-5, 1988. [PUBMED Abstract]
- Gershenson DM, Morris M, Cangir A, et al.: Treatment of malignant germ cell tumors of the ovary with bleomycin, etoposide, and cisplatin. J Clin Oncol 8 (4): 715-20, 1990. [PUBMED Abstract]
- Mitchell PL, Al-Nasiri N, A’Hern R, et al.: Treatment of nondysgerminomatous ovarian germ cell tumors: an analysis of 69 cases. Cancer 85 (10): 2232-44, 1999. [PUBMED Abstract]
- Dicken BJ, Billmire DF, Rich B, et al.: Utility of frozen section in pediatric and adolescent malignant ovarian nonseminomatous germ cell tumors: A report from the children’s oncology group. Gynecol Oncol 166 (3): 476-480, 2022. [PUBMED Abstract]
- Billmire DF, Cullen JW, Rescorla FJ, et al.: Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: report from the Children’s Oncology Group. J Clin Oncol 32 (5): 465-70, 2014. [PUBMED Abstract]
- Palenzuela G, Martin E, Meunier A, et al.: Comprehensive staging allows for excellent outcome in patients with localized malignant germ cell tumor of the ovary. Ann Surg 248 (5): 836-41, 2008. [PUBMED Abstract]
- Billmire D, Vinocur C, Rescorla F, et al.: Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. J Pediatr Surg 39 (3): 424-9; discussion 424-9, 2004. [PUBMED Abstract]
- Williams SD: Ovarian germ cell tumors: an update. Semin Oncol 25 (3): 407-13, 1998. [PUBMED Abstract]
- Williams S, Blessing JA, Liao SY, et al.: Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol 12 (4): 701-6, 1994. [PUBMED Abstract]
Treatment of Malignant Extragonadal Extracranial GCTs in Children
In initial reports, children with extragonadal malignant germ cell tumors (GCTs), particularly those with advanced-stage (stage III or stage IV) disease, had the highest risk of treatment failure for any GCT presentation.[1,2] Subsequently, an analysis of data from 25 years of pediatric GCT studies in the United States and United Kingdom reported that children younger than 11 years with extragonadal stage III and stage IV GCTs had an event-free survival (EFS) rate of 85%, and adolescents with stage III and stage IV extragonadal disease had poorer outcomes (expected EFS rate, <70%).[3]
The role of surgery at diagnosis for extragonadal tumors depends on patient age and tumor site, and treatment must be individualized. Depending on the clinical setting, the appropriate surgical approach may be primary resection, biopsy before chemotherapy, or no surgery (e.g., for a mediastinal primary tumor in a patient with a compromised airway and elevated tumor markers). An appropriate strategy may be biopsy at diagnosis followed by chemotherapy and subsequent surgery in selected patients who have residual masses after chemotherapy.
Standard Treatment Options for Malignant Extragonadal Extracranial GCTs in Prepubertal Children
Standard treatment options for malignant extragonadal extracranial GCTs in prepubertal children include the following:
The treatment of malignant extragonadal extracranial GCTs also depends on the site of disease. For more information, see the Site-specific considerations for malignant extragonadal extracranial GCTs section.
Surgery and chemotherapy (stages I–IV)
Surgery and chemotherapy with four cycles of standard cisplatin, etoposide, and bleomycin (PEb) is one treatment option. Patients with stage I and stage II disease treated with this regimen had an overall survival (OS) rate of 90%, suggesting that a reduction in therapy may be considered.[1,4] Patients with stage III and stage IV disease had OS rates of higher than 80%.[1]
An alternative treatment option is surgery and chemotherapy with carboplatin, etoposide, and bleomycin (JEb).[5] Stage III and stage IV patients treated with JEb had an OS rate similar to that with the PEb regimen.[5]
Two pediatric intergroup trials for patients with high-risk disease investigated the use of high-dose cisplatin (200 mg/m2) in a randomized study and a subsequent study that added amifostine to high-dose cisplatin.[1] No benefit in OS was observed, and 75% of patients required hearing aids. A Children’s Oncology Group (COG) trial of patients with high-risk disease investigated the addition of cyclophosphamide to standard-dose PEb. The addition of cyclophosphamide was feasible and well tolerated at all dose levels, but there was no evidence that adding cyclophosphamide improved efficacy.[6]
Biopsy followed by chemotherapy with or without surgery (stages III and IV)
While outcomes have improved remarkably since the advent of platinum-based chemotherapy and the use of a multidisciplinary treatment approach, complete resection before chemotherapy may be possible in some patients without major morbidity.[1,5]
However, for patients with locally advanced sacrococcygeal tumors, mediastinal tumors, or large pelvic tumors, tumor biopsy followed by preoperative chemotherapy may facilitate subsequent complete tumor resection and improve ultimate patient outcome. No decrease in OS has been noted for patients with malignant extragonadal GCTs who have had delayed resection after receiving chemotherapy.[5,7–9]
Site-specific considerations for malignant extragonadal extracranial GCTs
The treatment of malignant extragonadal extracranial GCTs depends in part on the site of disease.
Sacrococcygeal site
Sacrococcygeal GCTs are common extragonadal tumors that occur in very young children, predominantly young females.[10] The tumors are usually diagnosed at birth, when large external lesions predominate (usually mature or immature teratomas), or later in the first years of life, when presacral lesions with higher malignancy rates predominate.[10]
Malignant sacrococcygeal tumors are usually very advanced at diagnosis. Two-thirds of patients have locoregional disease, and metastases are present in 50% of patients.[8,11,12] Because of their advanced stage at presentation, the management of sacrococcygeal tumors requires a multimodal approach with platinum-based chemotherapy followed by delayed tumor resection.
Platinum-based therapies, with either cisplatin or carboplatin, are the cornerstone of treatment. The PEb regimen or the JEb regimen produces EFS rates of 85%.[8,9] Surgery may be facilitated by preoperative chemotherapy. In any patient with a sacrococcygeal GCT, resection of the coccyx is mandatory.[8,9]
Completeness of surgical resection is an important prognostic factor, as shown in the following circumstances:[8,9,13]
- Resected tumors with negative microscopic margins: EFS rates higher than 90%.
- Resected tumors with microscopic margins: EFS rates of 75% to 85%.
- Resected tumors with macroscopic residual disease: EFS rates lower than 40%.
Mediastinal site
Mediastinal GCTs account for 15% to 20% of malignant extragonadal extracranial GCTs in children.[5] The histology of mediastinal GCT is dependent on age, with teratomas predominating among infants and yolk sac tumors predominating among children aged 1 to 4 years.[7]
Prepubertal children with mediastinal malignant teratomas are treated with tumor resection, which is curative in almost all patients.[7] Children with stage I to stage III nonmetastatic mediastinal GCTs who receive cisplatin-based chemotherapy have 5-year EFS and OS rates of 90%. However, patients with stage IV mediastinal tumors have EFS rates closer to 80%.[3,5,7]; [14][Level of evidence C1]
Retroperitoneal site
Malignant GCTs located in the retroperitoneum or abdomen usually present in children younger than 5 years. Most of these tumors are advanced stage and locally unresectable at diagnosis.[15] A limited biopsy followed by platinum-based chemotherapy to shrink tumor bulk can lead to complete tumor resection in most patients. Despite the advanced-stage disease in most patients, the 6-year EFS rate using PEb was 83% in the Pediatric Oncology Group/Children’s Cancer Group intergroup study.[15]
Head and neck site
Although rare, benign and malignant GCTs can occur in the head and neck region, especially in infants. The airway is often threatened. Surgery for nonmalignant tumors and surgery plus chemotherapy for malignant tumors can be curative.[16][Level of evidence C2]
Standard Treatment Options for Malignant Extragonadal Extracranial GCTs in Postpubertal Children
In a study of prognostic factors in pediatric extragonadal malignant GCTs, age older than 12 years was the most important prognostic factor. In a multivariate analysis, children aged 12 years and older with thoracic tumors had six times the risk of death compared with children younger than 12 years with primary nonthoracic tumors.[17] In a subsequent meta-analysis, adolescents with stage III and stage IV extragonadal disease had poor outcomes (expected EFS rate, <70%).[3] Extragonadal disease of any stage is considered a poor risk factor in adolescents and young adults.[18]
Standard treatment options for malignant extragonadal extracranial GCTs in postpubertal children include the following:
- Surgery.
- Chemotherapy (four cycles of bleomycin, etoposide, and cisplatin [BEP]).
- Chemotherapy followed by surgery to remove residual tumor.
- Enrollment in a clinical trial.
As with sacrococcygeal tumors, primary chemotherapy is usually necessary to facilitate surgical resection of mediastinal GCTs, and the completeness of resection is a very important prognostic indicator.[7,19] Survival rates for the older adolescent and young adult population with mediastinal tumors are generally lower than 60%.[3,17,20–22]; [23][Level of evidence C1]
Patients with a malignant mediastinal primary tumor and extracranial metastases are at the highest risk of developing brain metastases and are monitored closely for signs and symptoms of central nervous system involvement.[24][Level of evidence C1] For more information about the treatment of adult patients, see Extragonadal Germ Cell Tumors Treatment.
Treatment Options Under Clinical Evaluation for Malignant Extragonadal Extracranial GCTs
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- AGCT1531 (NCT03067181) (Active Surveillance, Bleomycin, Carboplatin, Etoposide, or Cisplatin in Treating Pediatric and Adult Patients with GCTs): The aim of this trial is to reduce toxicity and maintain efficacy of treatment for patients with standard-risk GCTs. Patients with stage I malignant GCTs (low risk, age 0–50 years) will be treated with surgery and observation. Patients with intermediate-risk GCTs will be randomly assigned to receive cisplatin or carboplatin and bleomycin and etoposide. Children younger than 11 years will receive bleomycin with each cycle, and those aged 11 years and older will receive weekly bleomycin. Patients with pure seminoma or dysgerminoma are excluded from the trial.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Cushing B, Giller R, Cullen JW, et al.: Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol 22 (13): 2691-700, 2004. [PUBMED Abstract]
- Baranzelli MC, Kramar A, Bouffet E, et al.: Prognostic factors in children with localized malignant nonseminomatous germ cell tumors. J Clin Oncol 17 (4): 1212, 1999. [PUBMED Abstract]
- Frazier AL, Hale JP, Rodriguez-Galindo C, et al.: Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol 33 (2): 195-201, 2015. [PUBMED Abstract]
- Rogers PC, Olson TA, Cullen JW, et al.: Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study–Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J Clin Oncol 22 (17): 3563-9, 2004. [PUBMED Abstract]
- Mann JR, Raafat F, Robinson K, et al.: The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 18 (22): 3809-18, 2000. [PUBMED Abstract]
- Malogolowkin MH, Krailo M, Marina N, et al.: Pilot study of cisplatin, etoposide, bleomycin, and escalating dose cyclophosphamide therapy for children with high risk germ cell tumors: a report of the children’s oncology group (COG). Pediatr Blood Cancer 60 (10): 1602-5, 2013. [PUBMED Abstract]
- Schneider DT, Calaminus G, Reinhard H, et al.: Primary mediastinal germ cell tumors in children and adolescents: results of the German cooperative protocols MAKEI 83/86, 89, and 96. J Clin Oncol 18 (4): 832-9, 2000. [PUBMED Abstract]
- Göbel U, Schneider DT, Calaminus G, et al.: Multimodal treatment of malignant sacrococcygeal germ cell tumors: a prospective analysis of 66 patients of the German cooperative protocols MAKEI 83/86 and 89. J Clin Oncol 19 (7): 1943-50, 2001. [PUBMED Abstract]
- Rescorla F, Billmire D, Stolar C, et al.: The effect of cisplatin dose and surgical resection in children with malignant germ cell tumors at the sacrococcygeal region: a pediatric intergroup trial (POG 9049/CCG 8882). J Pediatr Surg 36 (1): 12-7, 2001. [PUBMED Abstract]
- Altman RP, Randolph JG, Lilly JR: Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 9 (3): 389-98, 1974. [PUBMED Abstract]
- Rescorla FJ, Sawin RS, Coran AG, et al.: Long-term outcome for infants and children with sacrococcygeal teratoma: a report from the Childrens Cancer Group. J Pediatr Surg 33 (2): 171-6, 1998. [PUBMED Abstract]
- Calaminus G, Schneider DT, Bökkerink JP, et al.: Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89. J Clin Oncol 21 (5): 781-6, 2003. [PUBMED Abstract]
- Egler RA, Gosiengfiao Y, Russell H, et al.: Is surgical resection and observation sufficient for stage I and II sacrococcygeal germ cell tumors? A case series and review. Pediatr Blood Cancer 64 (5): , 2017. [PUBMED Abstract]
- De Pasquale MD, Crocoli A, Conte M, et al.: Mediastinal Germ Cell Tumors in Pediatric Patients: A Report From the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer 63 (5): 808-12, 2016. [PUBMED Abstract]
- Billmire D, Vinocur C, Rescorla F, et al.: Malignant retroperitoneal and abdominal germ cell tumors: an intergroup study. J Pediatr Surg 38 (3): 315-8; discussion 315-8, 2003. [PUBMED Abstract]
- Bernbeck B, Schneider DT, Bernbeck B, et al.: Germ cell tumors of the head and neck: report from the MAKEI Study Group. Pediatr Blood Cancer 52 (2): 223-6, 2009. [PUBMED Abstract]
- Marina N, London WB, Frazier AL, et al.: Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study. J Clin Oncol 24 (16): 2544-8, 2006. [PUBMED Abstract]
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 15 (2): 594-603, 1997. [PUBMED Abstract]
- Billmire D, Vinocur C, Rescorla F, et al.: Malignant mediastinal germ cell tumors: an intergroup study. J Pediatr Surg 36 (1): 18-24, 2001. [PUBMED Abstract]
- Vuky J, Bains M, Bacik J, et al.: Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising from the mediastinum. J Clin Oncol 19 (3): 682-8, 2001. [PUBMED Abstract]
- Ganjoo KN, Rieger KM, Kesler KA, et al.: Results of modern therapy for patients with mediastinal nonseminomatous germ cell tumors. Cancer 88 (5): 1051-6, 2000. [PUBMED Abstract]
- Bokemeyer C, Nichols CR, Droz JP, et al.: Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 20 (7): 1864-73, 2002. [PUBMED Abstract]
- Kang CH, Kim YT, Jheon SH, et al.: Surgical treatment of malignant mediastinal nonseminomatous germ cell tumor. Ann Thorac Surg 85 (2): 379-84, 2008. [PUBMED Abstract]
- Göbel U, von Kries R, Teske C, et al.: Brain metastases during follow-up of children and adolescents with extracranial malignant germ cell tumors: risk adapted management decision tree analysis based on data of the MAHO/MAKEI-registry. Pediatr Blood Cancer 60 (2): 217-23, 2013. [PUBMED Abstract]
Treatment of Recurrent Malignant GCTs in Children
Only a small number of children and adolescents with extracranial germ cell tumors (GCTs) have a recurrence.[1,2] Reports regarding the treatment and outcome of these children are based on small studies.[3]
Treatment options for recurrent pediatric GCTs are modeled after treatment options in adult clinical trials. Information about ongoing National Cancer Institute (NCI)–supported clinical trials is available from the NCI website.
Standard Treatment Options for Recurrent Malignant GCTs in Children
Standard treatment options for recurrent childhood malignant GCTs include the following:
- Surgery alone.
- Surgery with neoadjuvant or adjuvant chemotherapy.
For information about salvage therapy after observation for patients with stage I disease, see the following sections:
- Surgery and Observation section of the Treatment Option Overview for Childhood Extracranial GCTs section.
- Surgery and observation (stage I) section of the Malignant testicular GCTs in prepubertal males section.
- Surgery and observation for prepubertal females (stage I following strict surgical staging guidelines) section of the Malignant nongerminomatous ovarian GCTs section.
Surgery with neoadjuvant or adjuvant chemotherapy
Reports of salvage treatment strategies used in adult recurrent GCTs include larger numbers of patients, but the differences between children and adults regarding the location of the primary GCT site, pattern of relapse, and the biology of childhood GCTs may limit the applicability of adult salvage approaches to children. In adults with recurrent GCTs, several chemotherapy combinations (most include the addition of paclitaxel and ifosfamide to a platinum compound) have achieved relatively good disease-free status.[4–9] A combination of paclitaxel and gemcitabine has demonstrated activity in adults with testicular GCTs who relapsed after high-dose chemotherapy and hematopoietic stem cell transplant (HSCT).[10]
Among children with benign sacrococcygeal tumors who recur, a malignant component may be present at the primary tumor site. For these children, complete surgical resection of the recurrent tumor and coccyx (if not done originally) is the basis of salvage treatment. Preoperative chemotherapy with cisplatin, etoposide, and bleomycin (PEb) may assist the surgical resection. In patients who had a malignant sacrococcygeal tumor that recurred after PEb treatment, surgery and additional chemotherapy may be warranted.[3]
In a phase II Children’s Oncology Group (COG) trial (AGCT0521 [NCT00467051]), 20 patients younger than 21 years who relapsed after PEb therapy received two cycles of paclitaxel, ifosfamide, and carboplatin (TIC). Responses were then assessed by a combination of Response Evaluation Criteria In Solid Tumors (RECIST) criteria and marker decline. Eight patients had partial responses, ten patients had stable disease, and two patients had progressive disease. This chemotherapy regimen produced a combined response rate of 44%.[11]
Nonstandard Treatment Options for Recurrent Malignant GCTs in Children
High-dose (HD) chemotherapy and hematopoietic stem cell rescue
The role of HD chemotherapy and hematopoietic stem cell rescue for recurrent pediatric GCTs is not established, despite anecdotal reports. In one European series, 10 of 23 children with relapsed extragonadal GCTs achieved long-term disease-free survival (median follow-up, 66 months) after receiving HD chemotherapy with stem cell support.[12] Additional study in children and adolescents is needed. For more information about transplant, see Pediatric Autologous Hematopoietic Stem Cell Transplant and Pediatric Hematopoietic Stem Cell Transplant and Cellular Therapy for Cancer.
HD chemotherapy with autologous stem cell rescue has been explored as a treatment for adults with recurrent testicular GCTs. HD chemotherapy plus hematopoietic stem cell rescue has been reported to cure adult patients with relapsed testicular GCTs, even as third-line therapy and in cisplatin-refractory patients.[10,13–15] A small study also demonstrated efficacy in adolescents and women with ovarian GCTs.[16][Level of evidence C1] While some studies support this approach,[10,14,15,17,18] others do not.[19,20] Salvage attempts using HD chemotherapy regimens may be of little benefit if the patient is not clinically disease free at the time of HSCT.[13,21]
Radiation therapy followed by surgery (for brain metastases)
In a very small pediatric study, patients with nongerminomatous brain metastases responded to radiation therapy. In the German Maligne Keimzelltümoren (MAKEI) studies, radiation therapy and surgery for patients with brain metastases provided palliation and occasional long-term survival.[22,23][Level of evidence C1] A meta-analysis showed that radiation therapy did not improve outcome compared with surgery and radiation. However, the number of patients treated with radiation therapy was too small to accurately assess outcome.[24]
Treatment Options Under Clinical Evaluation for Recurrent Malignant GCTs in Children and Adolescents
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, see the ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Mann JR, Raafat F, Robinson K, et al.: The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 18 (22): 3809-18, 2000. [PUBMED Abstract]
- Cushing B, Giller R, Cullen JW, et al.: Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol 22 (13): 2691-700, 2004. [PUBMED Abstract]
- Schneider DT, Wessalowski R, Calaminus G, et al.: Treatment of recurrent malignant sacrococcygeal germ cell tumors: analysis of 22 patients registered in the German protocols MAKEI 83/86, 89, and 96. J Clin Oncol 19 (7): 1951-60, 2001. [PUBMED Abstract]
- Loehrer PJ, Gonin R, Nichols CR, et al.: Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 16 (7): 2500-4, 1998. [PUBMED Abstract]
- Motzer RJ, Sheinfeld J, Mazumdar M, et al.: Paclitaxel, ifosfamide, and cisplatin second-line therapy for patients with relapsed testicular germ cell cancer. J Clin Oncol 18 (12): 2413-8, 2000. [PUBMED Abstract]
- Hartmann JT, Einhorn L, Nichols CR, et al.: Second-line chemotherapy in patients with relapsed extragonadal nonseminomatous germ cell tumors: results of an international multicenter analysis. J Clin Oncol 19 (6): 1641-8, 2001. [PUBMED Abstract]
- Kondagunta GV, Bacik J, Sheinfeld J, et al.: Paclitaxel plus Ifosfamide followed by high-dose carboplatin plus etoposide in previously treated germ cell tumors. J Clin Oncol 25 (1): 85-90, 2007. [PUBMED Abstract]
- Schmoll HJ, Kollmannsberger C, Metzner B, et al.: Long-term results of first-line sequential high-dose etoposide, ifosfamide, and cisplatin chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase I/II study of the German Testicular Cancer Study Group. J Clin Oncol 21 (22): 4083-91, 2003. [PUBMED Abstract]
- Kondagunta GV, Bacik J, Bajorin D, et al.: Etoposide and cisplatin chemotherapy for metastatic good-risk germ cell tumors. J Clin Oncol 23 (36): 9290-4, 2005. [PUBMED Abstract]
- Einhorn LH, Brames MJ, Juliar B, et al.: Phase II study of paclitaxel plus gemcitabine salvage chemotherapy for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. J Clin Oncol 25 (5): 513-6, 2007. [PUBMED Abstract]
- Pashankar F, Frazier AL, Krailo M, et al.: Treatment of refractory germ cell tumors in children with paclitaxel, ifosfamide, and carboplatin: A report from the Children’s Oncology Group AGCT0521 study. Pediatr Blood Cancer 65 (8): e27111, 2018. [PUBMED Abstract]
- De Giorgi U, Rosti G, Slavin S, et al.: Salvage high-dose chemotherapy for children with extragonadal germ-cell tumours. Br J Cancer 93 (4): 412-7, 2005. [PUBMED Abstract]
- Einhorn LH, Williams SD, Chamness A, et al.: High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 357 (4): 340-8, 2007. [PUBMED Abstract]
- Motzer RJ, Mazumdar M, Sheinfeld J, et al.: Sequential dose-intensive paclitaxel, ifosfamide, carboplatin, and etoposide salvage therapy for germ cell tumor patients. J Clin Oncol 18 (6): 1173-80, 2000. [PUBMED Abstract]
- Rick O, Bokemeyer C, Beyer J, et al.: Salvage treatment with paclitaxel, ifosfamide, and cisplatin plus high-dose carboplatin, etoposide, and thiotepa followed by autologous stem-cell rescue in patients with relapsed or refractory germ cell cancer. J Clin Oncol 19 (1): 81-8, 2001. [PUBMED Abstract]
- Meisel JL, Woo KM, Sudarsan N, et al.: Development of a risk stratification system to guide treatment for female germ cell tumors. Gynecol Oncol 138 (3): 566-72, 2015. [PUBMED Abstract]
- Bhatia S, Abonour R, Porcu P, et al.: High-dose chemotherapy as initial salvage chemotherapy in patients with relapsed testicular cancer. J Clin Oncol 18 (19): 3346-51, 2000. [PUBMED Abstract]
- Feldman DR, Sheinfeld J, Bajorin DF, et al.: TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol 28 (10): 1706-13, 2010. [PUBMED Abstract]
- Beyer J, Rick O, Siegert W, et al.: Salvage chemotherapy in relapsed germ cell tumors. World J Urol 19 (2): 90-3, 2001. [PUBMED Abstract]
- Beyer J, Kramar A, Mandanas R, et al.: High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables. J Clin Oncol 14 (10): 2638-45, 1996. [PUBMED Abstract]
- Rick O, Bokemeyer C, Weinknecht S, et al.: Residual tumor resection after high-dose chemotherapy in patients with relapsed or refractory germ cell cancer. J Clin Oncol 22 (18): 3713-9, 2004. [PUBMED Abstract]
- Göbel U, von Kries R, Teske C, et al.: Brain metastases during follow-up of children and adolescents with extracranial malignant germ cell tumors: risk adapted management decision tree analysis based on data of the MAHO/MAKEI-registry. Pediatr Blood Cancer 60 (2): 217-23, 2013. [PUBMED Abstract]
- Göbel U, Schneider DT, Teske C, et al.: Brain metastases in children and adolescents with extracranial germ cell tumor – data of the MAHO/MAKEI-registry. Klin Padiatr 222 (3): 140-4, 2010. [PUBMED Abstract]
- Feldman DR, Lorch A, Kramar A, et al.: Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options–An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol 34 (4): 345-51, 2016. [PUBMED Abstract]
Latest Updates to This Summary (11/05/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood extracranial germ cell tumors. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Extracranial Germ Cell Tumors Treatment are:
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children’s Healthcare of Atlanta – Egleston Campus)
- D. Williams Parsons, MD, PhD (Texas Children’s Hospital)
- Stephen J. Shochat, MD (St. Jude Children’s Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Extracranial Germ Cell Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: /types/extracranial-germ-cell/hp/germ-cell-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389316]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.