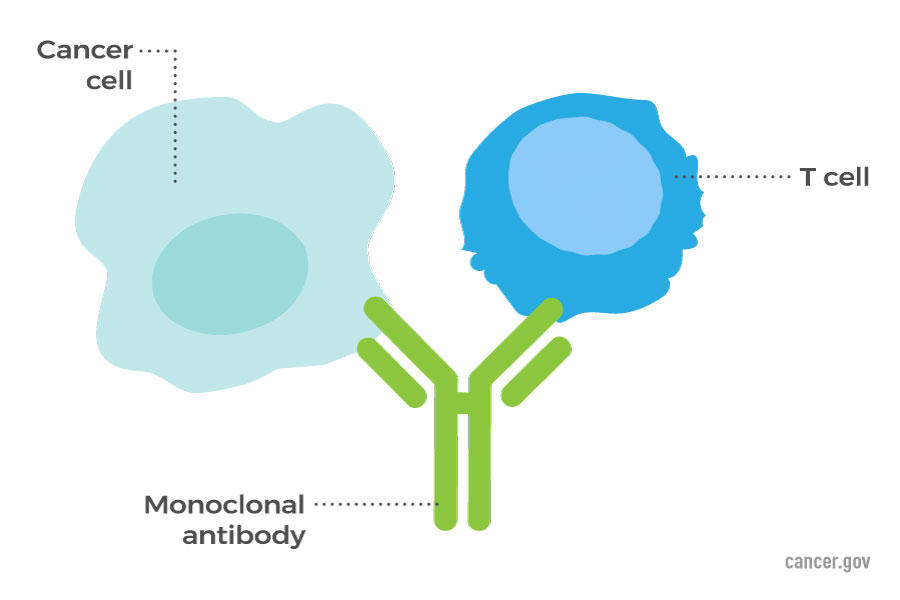
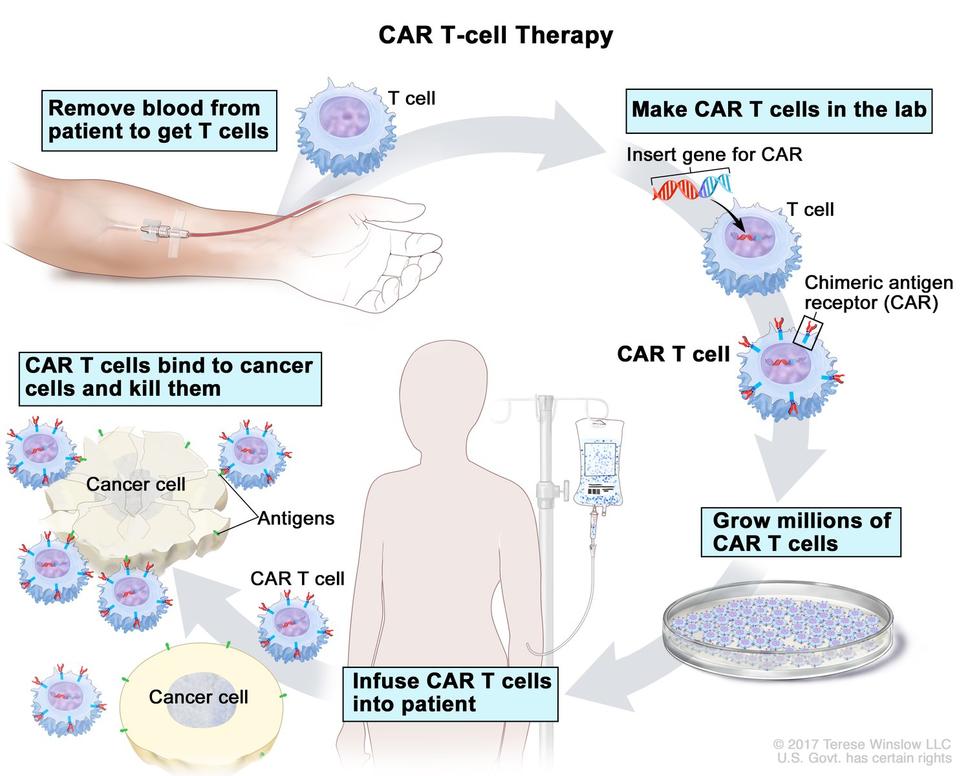
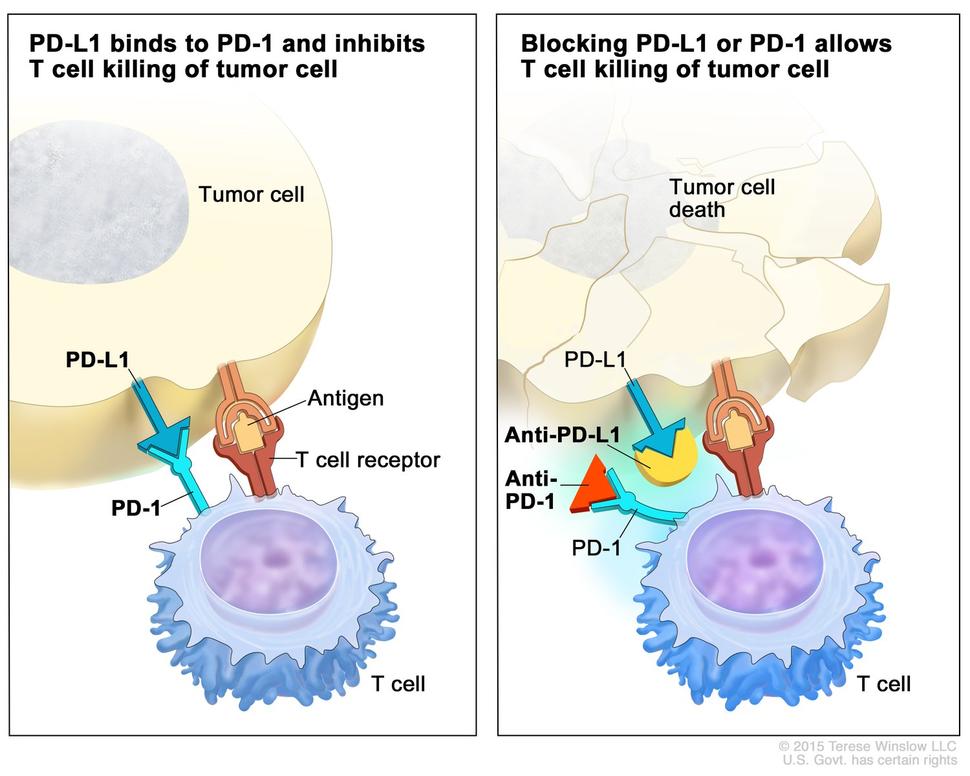
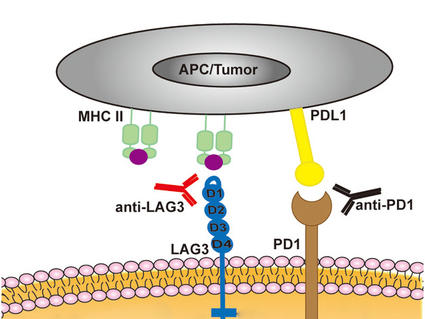
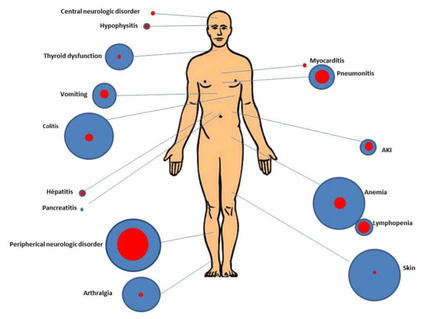
Kidney (Renal Cell) Cancer—Patient Version
Overview
Kidney cancer can develop in adults and children. The main types of kidney cancer are renal cell cancer, transitional cell cancer, and Wilms tumor. Certain inherited conditions increase the risk of kidney cancer. Explore the links on this page to learn more about kidney cancer treatment, statistics, research, and clinical trials.
Treatment
PDQ Treatment Information for Patients
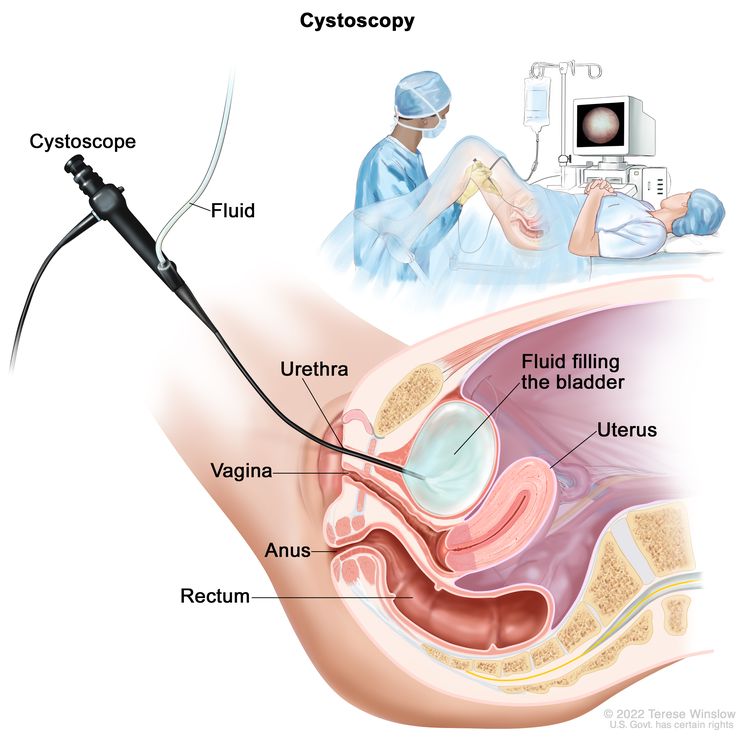
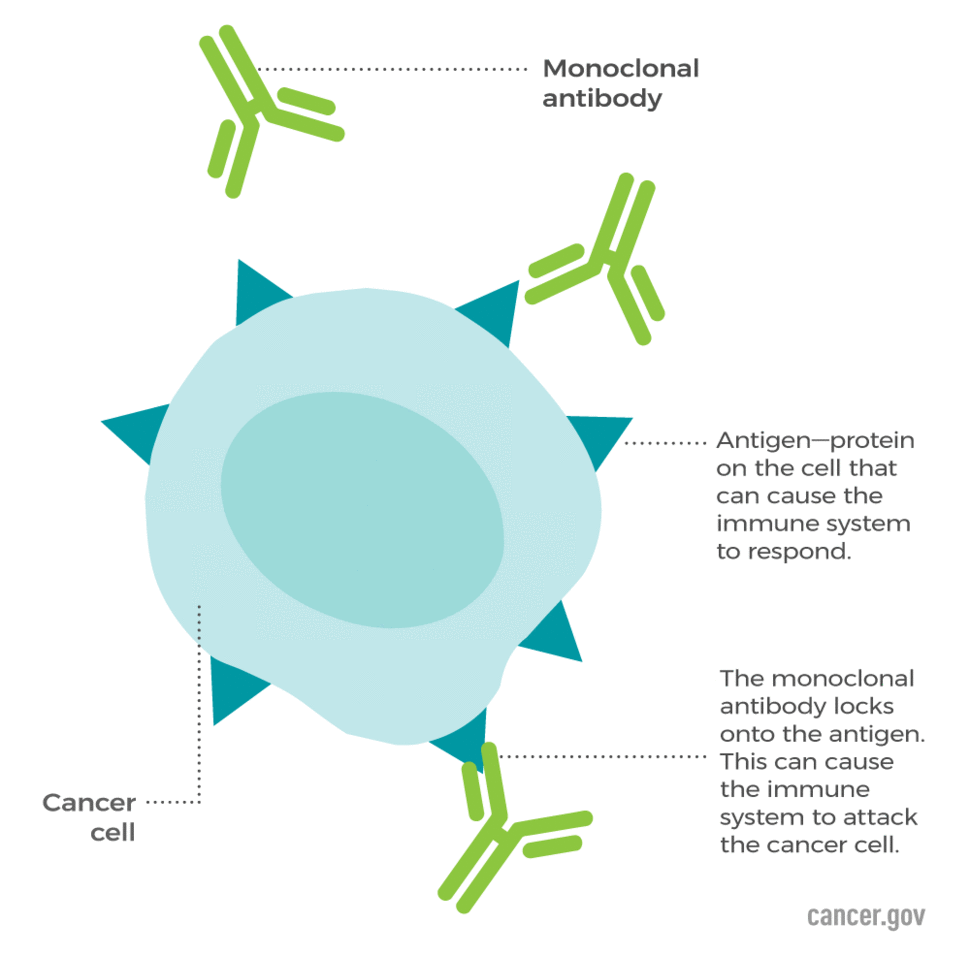
- Renal Cell Cancer Treatment
- Transitional Cell Cancer (Kidney/Ureter) Treatment
- Wilms Tumor
- Kidney Cancer in Children
More information
Causes & Prevention
NCI does not have PDQ evidence-based information about prevention of kidney cancer.
More information
Genetics
PDQ Genetics Information for Patients
More information
Screening
NCI does not have PDQ evidence-based information about screening for kidney cancer.
More information
Coping with Cancer
The information in this section is meant to help you cope with the many issues and concerns that occur when you have cancer.
Emotions and Cancer Adjusting to Cancer Support for Caregivers Survivorship Advanced Cancer Managing Cancer Care