Anal Cancer Treatment (PDQ®)–Health Professional Version
General Information About Anal Cancer
Incidence and Mortality
Estimated new cases and deaths from anal, anal canal, and anorectal cancer in the United States in 2025:[1]
- New cases: 10,930.
- Deaths: 2,030.
Prognosis and Survival
The two major prognostic factors for anal cancer are tumor size and nodal status. Primary tumors smaller than 2 cm have a better prognosis.[2] Nodal drainage of the anus follows the inguinal vein. The initial evaluation of a patient with anal cancer will include a careful clinical examination of the inguinal region and biopsy of any palpable lymph nodes. For more information, see the American Joint Committee on Cancer Stage Groupings and TNM Definitions section.
Anal cancer is usually curable. At presentation, most patients have T1 or T2 disease (≤5 cm), and fewer than 20% of patients have node-positive disease. The 5-year survival rate for these early-stage patients exceeds 85%.[3,4] Even in patients with node-positive disease, 5-year survival rates exceed 50% in the absence of invasion into adjacent organs or distant metastases.[5]
Risk Factors
Overall, the risk of anal cancer is rising due to increased incidence of human papillomavirus (HPV) infection.[6,7] Ninety-five percent of anal cancers are HPV related, with the highest risk for serotypes 16 and 18. Involvement of HPV can be pathologically correlated with P16+ staining.[8] Patients with HIV have a higher risk of HPV coinfection, and consequently have a higher risk of anal cancer.
Data suggest that certain sexual practices, such as receptive anal intercourse or a high lifetime number of sexual partners, portend an increased risk of anal cancer. These practices may have led to an increase in the number of individuals at risk of infection with HPV.[6]
References
- American Cancer Society: Cancer Facts and Figures 2025. American Cancer Society, 2025. Available online. Last accessed January 16, 2025.
- Ajani JA, Winter KA, Gunderson LL, et al.: Prognostic factors derived from a prospective database dictate clinical biology of anal cancer: the intergroup trial (RTOG 98-11). Cancer 116 (17): 4007-13, 2010. [PUBMED Abstract]
- Klas JV, Rothenberger DA, Wong WD, et al.: Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer 85 (8): 1686-93, 1999. [PUBMED Abstract]
- Touboul E, Schlienger M, Buffat L, et al.: Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer 73 (6): 1569-79, 1994. [PUBMED Abstract]
- Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 275–84.
- Johnson LG, Madeleine MM, Newcomer LM, et al.: Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer 101 (2): 281-8, 2004. [PUBMED Abstract]
- Holly EA, Ralston ML, Darragh TM, et al.: Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst 93 (11): 843-9, 2001. [PUBMED Abstract]
- Ryan DP, Compton CC, Mayer RJ: Carcinoma of the anal canal. N Engl J Med 342 (11): 792-800, 2000. [PUBMED Abstract]
Cellular Classification of Anal Cancer
Squamous cell (epidermoid) carcinomas make up most primary anal cancers. Historically, a subset of tumors arising from the epithelial transitional zone were categorized as cloacogenic or basaloid tumors. However, these tumors are now recognized as nonkeratinizing squamous cell cancers and are similarly associated with human papillomavirus.[1,2]
Lesions in the hair-bearing skin distal to the squamous mucocutaneous junction are defined as perianal cancers. These are typically treated the same as anal canal cancers, although local therapy alone can be considered for discrete skin lesions with significant separation from the anal verge.
Adenocarcinomas starting in anal glands or fistulae formation are rare and generally have clinical features that are similar to rectal adenocarcinoma. For more information, see the Clinical Features section in Rectal Cancer Treatment.
Treatment of anal melanoma is not included in this summary.
References
- Palefsky JM, Holly EA, Gonzales J, et al.: Detection of human papillomavirus DNA in anal intraepithelial neoplasia and anal cancer. Cancer Res 51 (3): 1014-9, 1991. [PUBMED Abstract]
- Pirog EC, Quint KD, Yantiss RK: P16/CDKN2A and Ki-67 enhance the detection of anal intraepithelial neoplasia and condyloma and correlate with human papillomavirus detection by polymerase chain reaction. Am J Surg Pathol 34 (10): 1449-55, 2010. [PUBMED Abstract]
Stage Information for Anal Cancer
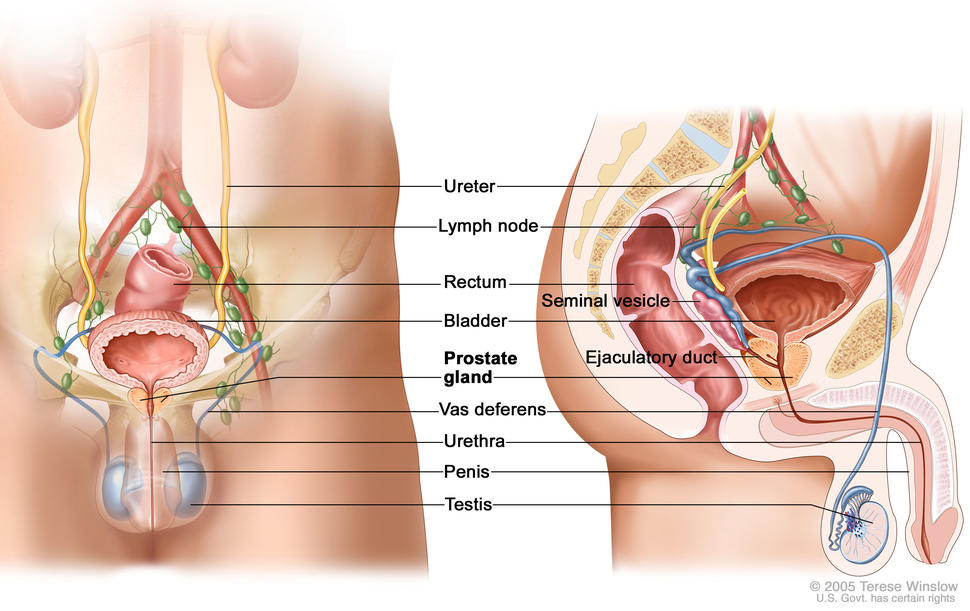
The anal canal extends from the rectum to the perianal skin and is lined by a mucous membrane that covers the internal sphincter. Tumors of the anal margin (below the anal verge and involving the perianal hair-bearing skin) are classified with skin tumors.
American Joint Committee on Cancer (AJCC) Stage Groupings and TNM Definitions
The following is a staging system for anal canal cancer that has been described by the AJCC and the International Union Against Cancer.[1] The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define anal cancer.
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 275–84. | ||
| 0 | Tis, N0, M0 | Tis = High-grade squamous intraepithelial lesion (previously termed carcinoma in situ, Bowen disease, anal intraepithelial neoplasia II–III, high-grade anal intraepithelial neoplasia). |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 275–84. | ||
| I | T1, N0, M0 | T1 = Tumor ≤2 cm. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 275–84. | ||
| IIA | T2, N0, M0 | T2 = Tumor >2 cm but ≤5 cm. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| IIB | T3, N0, M0 | T3 = Tumor >5 cm. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 275–84. | ||
| IIIA | T1, N1, M0 | T1 = Tumor ≤2 cm. |
| N1 = Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes. | ||
| M0 = No distant metastasis. | ||
| T2, N1, M0 | T2 = Tumor >2 cm but ≤5 cm. | |
| N1 = Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes. | ||
| M0 = No distant metastasis. | ||
| IIIB | T4, N0, M0 | T4 = Tumor of any size invading adjacent organ(s), such as the vagina, urethra, or bladder. |
| N0 = No regional lymph node metastasis. | ||
| M0 = No distant metastasis. | ||
| IIIC | T3, N1, M0 | T3 = Tumor >5 cm. |
| N1 = Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes. | ||
| M0 = No distant metastasis. | ||
| T4, N1, M0 | T4 = Tumor of any size invading adjacent organ(s), such as the vagina, urethra, or bladder. | |
| N1 = Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes. | ||
| M0 = No distant metastasis. | ||
| Stage | TNM | Description |
|---|---|---|
| T = primary tumor; N = regional lymph node; M = distant metastasis. | ||
| aReprinted with permission from AJCC: Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 275–84. | ||
| IV | Any T, Any N, M1 | TX = Primary tumor not assessed. |
| T0 = No evidence of primary tumor. | ||
| Tis = High-grade squamous intraepithelial lesion (previously termed carcinoma in situ, Bowen disease, anal intraepithelial neoplasia II–III, high-grade anal intraepithelial neoplasia). | ||
| T1 = Tumor ≤2 cm. | ||
| T2 = Tumor >2 cm but ≤5 cm. | ||
| T3 = Tumor >5 cm. | ||
| T4 = Tumor of any size invading adjacent organ(s), such as the vagina, urethra, or bladder. | ||
| NX = Regional lymph nodes cannot be assessed. | ||
| N0 = No regional lymph node metastasis. | ||
| N1 = Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes. | ||
| –N1a = Metastasis in inguinal, mesorectal, or internal iliac lymph nodes. | ||
| –N1b = Metastasis in external iliac lymph nodes. | ||
| –N1c = Metastasis in external iliac with any N1a nodes. | ||
| M1 = Distant metastasis. | ||
References
- Anus. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 275–84.
Treatment Option Overview for Anal Cancer
Treatment options for anal cancer are described in Table 6.
| Stage (TNM Staging Criteria) | Treatment Options |
|---|---|
| Stage 0 | Surgery |
| Stages I, II, and III | Local resection |
| External-beam radiation therapy with chemotherapy | |
| Alternative strategies | |
| Radical resection | |
| Stage IV | Palliative surgery |
| Palliative radiation therapy | |
| Palliative chemotherapy (with or without radiation therapy) | |
| Checkpoint inhibitors |
The optimal approach in patients with advanced disease is still under clinical evaluation. Information about ongoing clinical trials is available from the NCI website.
Capecitabine and Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[1,2] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[1–3] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient’s DPYD genotype and number of functioning DPYD alleles.[4–6] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[7] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[8]
References
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PUBMED Abstract]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PUBMED Abstract]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PUBMED Abstract]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PUBMED Abstract]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PUBMED Abstract]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PUBMED Abstract]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PUBMED Abstract]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PUBMED Abstract]
Treatment of Stage 0 Anal Cancer
Treatment Options for Stage 0 Anal Cancer
Stage 0 anal cancer is carcinoma in situ. Rarely diagnosed, it is a very early cancer that has not spread below the limiting membrane of the first layer of anal tissue.
Treatment options for stage 0 anal cancer include:
- Surgical resection is used to treat lesions of the perianal area not involving the anal sphincter. The surgical approach depends on the location of the lesion in the anal canal.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of Stages I, II, and III Anal Cancer
Treatment Options for Stages I, II, and III Anal Cancer
Current sphincter-sparing therapies include wide local excision for small tumors of the perianal skin or anal margin, or definitive chemoradiation therapy (fluorouracil [5-FU] and mitomycin) for cancers of the anal canal. Radical resection is reserved for patients with incomplete responses or recurrent disease.
Continued surveillance with rectal examination every 3 months for the first 2 years and endoscopy with biopsy when indicated after completion of sphincter-preserving therapy is important to monitor for recurrence.
Treatment options for stage I, stage II, and stage III anal cancer include:
- Small tumors of the perianal skin or anal margin not involving the anal sphincter may be adequately treated with local resection.[1]
- The standard of care for all other stage I, II, and III anal cancers in appropriate patients is chemoradiation therapy (external-beam radiation therapy [EBRT] with chemotherapy).
- Alternative strategies such as radiation therapy alone or surgery alone may be considered, depending on the clinical context.
- Radical resection is reserved for residual or recurrent cancer in the anal canal after nonoperative therapy.
Chemoradiation therapy
Because of historically high rates of recurrence with colostomy alone, chemoradiation therapy is the preferred approach for patients with anal cancer in the absence of distant metastases.
Evidence (chemoradiation therapy):
- The Anal Cancer Trial (ACT I) from the United Kingdom Co-ordinating Committee on Cancer Research demonstrated the superiority of chemoradiation with 5-FU and mitomycin over radiation therapy alone with regard to local failure and deaths from anal cancer.[2,8][Level of evidence A1]
In this prospective trial, 585 patients were randomly assigned to receive 45 Gy of radiation in 20 or 25 fractions with or without 5-FU. The 5-FU was given by continuous infusion (750 mg/m2 for 5 days or 1,000 mg/m2 for 4 days) during the first and final weeks of radiation therapy, along with a single dose of mitomycin (12 mg/m2) on the first day.
- After a median follow-up of 13.1 years, patients who received chemoradiation therapy had a reduction in local failure (36% vs. 59%; hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.35−0.60; P < .001), risk of death from anal cancer (HR, 0.61; 95% CI, 0.49−0.76; P < .001), and relapse at 12 years (17.7% vs. 29.7%; HR, 0.70; 95% CI, 9.58−0.84; P < .001).[2][Level of evidence A1]
- There was no significant difference in overall survival (OS) in this trial (HR, 0.86; 95% CI, 0.70−1.04; P = .12).
- An initial 9.1% increase in non–anal cancer deaths was observed in the first 5 years after chemoradiation therapy but was not seen at 10 years.
- A European Organisation for Research and Treatment of Cancer (EORTC) trial prospectively randomly assigned 100 patients with T3 to T4 or N1 to N3 disease to receive 45 Gy of radiation with a 15-Gy or 30-Gy boost with or without 5-FU infusion (750 mg/m2 for 5 days starting on days 1 and 29) plus mitomycin (15 mg/m2 on day 1).[3][Level of evidence B1]
- Outcomes favored chemoradiation therapy with respect to 5-year colostomy-free survival rates (75% vs. 48%; P = .002) and 5-year progression-free survival (PFS) rates (60% vs. 48%; P = .05).
Subsequent trials have found capecitabine to be a reasonable replacement for 5-FU in combination with mitomycin and radiation therapy.[4,5]
While the ACT I and EORTC randomized trials established chemoradiation therapy as the preferred approach for nonmetastatic anal cancer, the substantial hematological, renal, and pulmonary toxicity of mitomycin has prompted studies of alternative regimens.
Evidence (chemoradiation therapy [alternative regimens]):
- A Radiation Therapy Oncology Group (RTOG)/Eastern Cooperative Oncology Group trial of 310 patients studied chemoradiation therapy (5-FU infusion + 45 Gy of radiation) with or without mitomycin.
- After 4-years of follow-up, patients who received mitomycin had an improved colostomy-free survival rate (71% vs. 59%; P = .014) and disease-free survival (DFS) rate (73% vs. 51%; P = .0003).[9][Level of evidence B1]
Two large intergroup trials studied the substitution of cisplatin for mitomycin, with differing conclusions.
- In a phase III U.S. Intergroup trial (RTOG 9811 [NCT00003596]), patients in the cisplatin arm received two cycles of induction 5-FU and cisplatin before receiving concurrent chemoradiation therapy with 5-FU and cisplatin.[6]
- Patients who received mitomycin had improved local control and an improved colostomy-free survival rate (90% vs. 81%; P = .02). Subsequent long-term follow-up demonstrated a borderline significant difference in the 5-year colostomy-free survival rate (71.9% vs. 65%; P = .05).[10]
- Long-term follow-up also demonstrated a superior 5-year DFS rate (67.8% vs. 57.8%; P = .006) and OS rate (78.3% vs. 70.7%; P = .074) for patients who received mitomycin.[11][Level of evidence B1]
- One potential explanation for the inferiority of cisplatin in this study was the delay in time to radiation therapy during induction chemotherapy.
- In the prospective randomized ACT II trial, 940 patients were assigned in a 2 × 2 factorial design to receive the following: (1) either mitomycin or cisplatin during induction chemoradiation therapy and (2) either maintenance therapy with 5-FU and cisplatin in weeks 11 and 14 or no maintenance therapy.[7]
- The complete remission rate was equivalent in patients who received mitomycin or cisplatin after a median follow-up of 5.1 years (90.5% vs. 89.6%; 95% CI, -4.9 to 3.1; P = .64). The 3-year PFS rate was also equivalent in both study groups (73% for mitomycin vs. 72% for cisplatin; HR, 0.95; 95% CI, 0.75−1.19; P = .063).[7][Level of evidence B1]
- There was also no significant effect on 3-year PFS rates among patients who received maintenance therapy or no maintenance therapy (74% vs. 73%; HR, 0.95; 95% CI, 0.75−1.21; P = .70).
- This study suggests that cisplatin might reasonably substitute for mitomycin in a chemoradiation strategy.
The best time to assess a complete clinical response after chemoradiation therapy is generally after 26 weeks because delayed responses are seen.[12] Residual disease or subsequent local recurrence require further treatment.
The standard salvage therapy for patients with either gross or microscopic residual disease after chemoradiation therapy has been abdominoperineal resection. Alternatively, patients may be treated with additional salvage chemoradiation therapy, chemotherapy alone, or immunotherapy.[12,13]
The optimal radiation dose in various situations has not been determined. There is insufficient evidence to determine whether the dose should be escalated for patients with T3 to T4 disease or nodal metastases, or potentially de-escalated for patients with early-stage tumors smaller than 1 cm. It is also unclear whether the chemotherapy backbone can be safely omitted for some patients with early-stage tumors, and whether such a strategy would affect the optimal dose of radiation. The roles for newer strategies such as intensity-modulated radiation therapy, proton beam therapy, and brachytherapy have yet to be conclusively determined.[14–16] Based on the National Cancer Database, higher volume radiation oncology centers report improved OS for patients with anal cancer.[17]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Enker WE, Heilwell M, Janov AJ, et al.: Improved survival in epidermoid carcinoma of the anus in association with preoperative multidisciplinary therapy. Arch Surg 121 (12): 1386-90, 1986. [PUBMED Abstract]
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al.: Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 102 (7): 1123-8, 2010. [PUBMED Abstract]
- Bartelink H, Roelofsen F, Eschwege F, et al.: Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 15 (5): 2040-9, 1997. [PUBMED Abstract]
- Goodman KA, Julie D, Cercek A, et al.: Capecitabine With Mitomycin Reduces Acute Hematologic Toxicity and Treatment Delays in Patients Undergoing Definitive Chemoradiation Using Intensity Modulated Radiation Therapy for Anal Cancer. Int J Radiat Oncol Biol Phys 98 (5): 1087-1095, 2017. [PUBMED Abstract]
- Meulendijks D, Dewit L, Tomasoa NB, et al.: Chemoradiotherapy with capecitabine for locally advanced anal carcinoma: an alternative treatment option. Br J Cancer 111 (9): 1726-33, 2014. [PUBMED Abstract]
- Ajani JA, Winter KA, Gunderson LL, et al.: Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 299 (16): 1914-21, 2008. [PUBMED Abstract]
- James RD, Glynne-Jones R, Meadows HM, et al.: Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 14 (6): 516-24, 2013. [PUBMED Abstract]
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 348 (9034): 1049-54, 1996. [PUBMED Abstract]
- Flam M, John M, Pajak TF, et al.: Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 14 (9): 2527-39, 1996. [PUBMED Abstract]
- Eng C, Ciombor KK, Cho M, et al.: Anal Cancer: Emerging Standards in a Rare Disease. J Clin Oncol 40 (24): 2774-2788, 2022. [PUBMED Abstract]
- Gunderson LL, Winter KA, Ajani JA, et al.: Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 30 (35): 4344-51, 2012. [PUBMED Abstract]
- Pedersen TB, Gocht-Jensen P, Klein MF: 30-day and long-term outcome following salvage surgery for squamous cell carcinoma of the anus. Eur J Surg Oncol 44 (10): 1518-1521, 2018. [PUBMED Abstract]
- Guerra GR, Kong JC, Bernardi MP, et al.: Salvage Surgery for Locoregional Failure in Anal Squamous Cell Carcinoma. Dis Colon Rectum 61 (2): 179-186, 2018. [PUBMED Abstract]
- Cordoba A, Escande A, Leroy T, et al.: Low-dose-rate interstitial brachytherapy boost for the treatment of anal canal cancers. Brachytherapy 16 (1): 230-235, 2017 Jan – Feb. [PUBMED Abstract]
- Call JA, Prendergast BM, Jensen LG, et al.: Intensity-modulated Radiation Therapy for Anal Cancer: Results From a Multi-Institutional Retrospective Cohort Study. Am J Clin Oncol 39 (1): 8-12, 2016. [PUBMED Abstract]
- Gryc T, Ott O, Putz F, et al.: Interstitial brachytherapy as a boost to patients with anal carcinoma and poor response to chemoradiation: Single-institution long-term results. Brachytherapy 15 (6): 865-872, 2016 Nov – Dec. [PUBMED Abstract]
- Amini A, Jones BL, Ghosh D, et al.: Impact of facility volume on outcomes in patients with squamous cell carcinoma of the anal canal: Analysis of the National Cancer Data Base. Cancer 123 (2): 228-236, 2017. [PUBMED Abstract]
Treatment of Stage IV Anal Cancer
Treatment Options for Stage IV Anal Cancer
Treatment options for stage IV anal cancer include:
- Palliative surgery.
- Palliative radiation therapy.
- Palliative chemotherapy (with or without radiation therapy).
- Checkpoint inhibitors.
Advanced-stage therapy
- In the multicenter, randomized, phase II International Advanced Anal Cancer InterAACT trial (NCT02560298), carboplatin (area under the curve 5) and weekly paclitaxel was compared with standard infusional 5-FU and bolus cisplatin in patients with advanced-stage anal cancer.[2]
- With a median follow-up of 25.3 months, the median overall survival (OS) with carboplatin and paclitaxel was improved compared with cisplatin and 5-FU (20 months vs. 12.3 months; hazard ratio [HR], 2.0; P = .014).[Level of evidence A1]
- Serious adverse events were more common in patients treated with cisplatin plus 5-FU (62% vs. 36%; P = .016).
These promising findings have led international investigators to use carboplatin and paclitaxel as a new backbone in trials for patients with advanced-stage disease, as well as a potential partner for use with radiation therapy. Other chemotherapy regimens, such as modified docetaxel, cisplatin, and 5-FU, are under clinical evaluation.[3]
- The checkpoint inhibitors have also shown activity for patients with metastatic disease. The phase II NCI96773 trial (NCT02314169) of single-agent nivolumab (3 mg/kg every 2 weeks) enrolled 37 patients.[4]
- The overall response rate was 24%, including two complete responses.[4][Level of evidence C3]
- The phase Ib KEYNOTE-028 trial (NCT02054806) for patients with advanced tumors with programmed death ligand-1 of at least 1% enrolled a cohort of 24 patients with anal squamous cell carcinoma.[5]
- The overall response rate was 17%, and an additional stable disease rate was 42%.[5][Level of evidence C3]
Although there is no clear standard of care for patients with metastatic disease, recent studies are uncovering promising new avenues for systemic treatment. Palliation of symptoms from the primary lesion is important. Patients with stage IV disease should strongly consider enrolling in clinical trials.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- James RD, Glynne-Jones R, Meadows HM, et al.: Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 14 (6): 516-24, 2013. [PUBMED Abstract]
- Rao S, Sclafani F, Eng C, et al.: International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. J Clin Oncol 38 (22): 2510-2518, 2020. [PUBMED Abstract]
- Kim S, François E, André T, et al.: Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol 19 (8): 1094-1106, 2018. [PUBMED Abstract]
- Morris VK, Salem ME, Nimeiri H, et al.: Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 18 (4): 446-453, 2017. [PUBMED Abstract]
- Ott PA, Piha-Paul SA, Munster P, et al.: Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol 28 (5): 1036-1041, 2017. [PUBMED Abstract]
Treatment of HIV and Anal Cancer
The tolerance of patients with HIV and anal carcinoma to standard fluorouracil and mitomycin chemoradiation therapy is not well defined.[1,2] In general, patients with HIV are treated similarly to other patients and have similar outcomes, particularly in the era of highly active antiretroviral therapy (HAART). Patients with pretreatment CD4 counts of fewer than 200 cells/μl may have increased acute and late toxic effects.[3,4] Therefore, patients with a history of AIDS-related complications may have difficulty tolerating a standard regimen, necessitating a dose adjustment or omission of mitomycin.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Holland JM, Swift PS: Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology 193 (1): 251-4, 1994. [PUBMED Abstract]
- Peddada AV, Smith DE, Rao AR, et al.: Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 37 (5): 1101-5, 1997. [PUBMED Abstract]
- Hoffman R, Welton ML, Klencke B, et al.: The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys 44 (1): 127-31, 1999. [PUBMED Abstract]
- Place RJ, Gregorcyk SG, Huber PJ, et al.: Outcome analysis of HIV-positive patients with anal squamous cell carcinoma. Dis Colon Rectum 44 (4): 506-12, 2001. [PUBMED Abstract]
Treatment of Recurrent Anal Cancer
Local recurrences and persistent disease after treatment with radiation therapy and chemotherapy or surgery as the primary treatment may be controlled by using the alternate treatment (surgical resection after radiation and vice versa).[1] Salvage chemoradiation therapy with fluorouracil and cisplatin plus a radiation boost may avoid permanent colostomy in patients with residual tumor after initial nonoperative therapy.[2] Clinical trials are exploring the use of radiation therapy with chemotherapy and radiosensitizers to improve local control.
Preliminary studies in patients with stage IV disease suggest that alternative chemotherapy regimens (such as carboplatin and paclitaxel in the InterACCT trial [NCT02560298]) or immune checkpoint inhibitors (as in NCI9673 [NCT02314169] and KEYNOTE-028 [NCT02054806]) may be beneficial in this setting.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Longo WE, Vernava AM, Wade TP, et al.: Recurrent squamous cell carcinoma of the anal canal. Predictors of initial treatment failure and results of salvage therapy. Ann Surg 220 (1): 40-9, 1994. [PUBMED Abstract]
- Flam M, John M, Pajak TF, et al.: Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 14 (9): 2527-39, 1996. [PUBMED Abstract]
Latest Updates to This Summary (02/12/2025)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Anal Cancer
Updated statistics with estimated new cases and deaths for 2025 (cited American Cancer Society as reference 1).
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of anal cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Anal Cancer Treatment are:
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Leon Pappas, MD, PhD (Massachusetts General Hospital)
- Ari Seifter, MD (Advocate Health Care)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Anal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: /types/anal/hp/anal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389221]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.