HIV Infection and Cancer Risk
Do people infected with human immunodeficiency virus (HIV) have an increased risk of cancer?
Yes. People infected with HIV have a substantially higher risk of some types of cancer compared with uninfected people of the same age (1). The general term for these cancers is “HIV-associated cancers.” Three of these cancers are known as “acquired immunodeficiency syndrome (AIDs)-defining cancers” or “AIDS-defining malignancies”: Kaposi sarcoma, aggressive B-cell non-Hodgkin lymphoma, and cervical cancer. A diagnosis of any of these cancers in someone infected with HIV confirms a diagnosis of AIDS.
Compared with the general population, people infected with HIV are currently about 500 times more likely to be diagnosed with Kaposi sarcoma, 12 times more likely to be diagnosed with non-Hodgkin lymphoma, and, among women, 3 times more likely to be diagnosed with cervical cancer (2).
In addition, people infected with HIV are at higher risk of several other types of cancer (collectively called “non–AIDS-defining cancers“) (1, 2). These other malignancies include cancers of the anus, liver, oral cavity/pharynx, and lung, and Hodgkin lymphoma (3, 4).
People infected with HIV are 19 times more likely to be diagnosed with anal cancer, 3 times as likely to be diagnosed with liver cancer, 2 times as likely to be diagnosed with lung cancer, about 2 times as likely to be diagnosed with oral cavity/pharynx cancer, and about 8 times more likely to be diagnosed with Hodgkin lymphoma compared with the general population (2).
In addition to being linked to an increased risk of cancer, HIV infection is associated with an increased risk of dying from cancer. HIV-infected people with a range of cancer types are more likely to die of their cancer than HIV-uninfected people with these cancers (5, 6).
Why might people infected with HIV have a higher risk of some types of cancer?
Infection with HIV weakens the immune system and reduces the body’s ability to fight viral infections that may lead to cancer (2, 7, 8). The viruses that are most likely to cause cancer in people with HIV are (9):
- Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), which causes Kaposi sarcoma and some subtypes of lymphoma
- Epstein-Barr virus (EBV), which causes some subtypes of non-Hodgkin and Hodgkin lymphoma
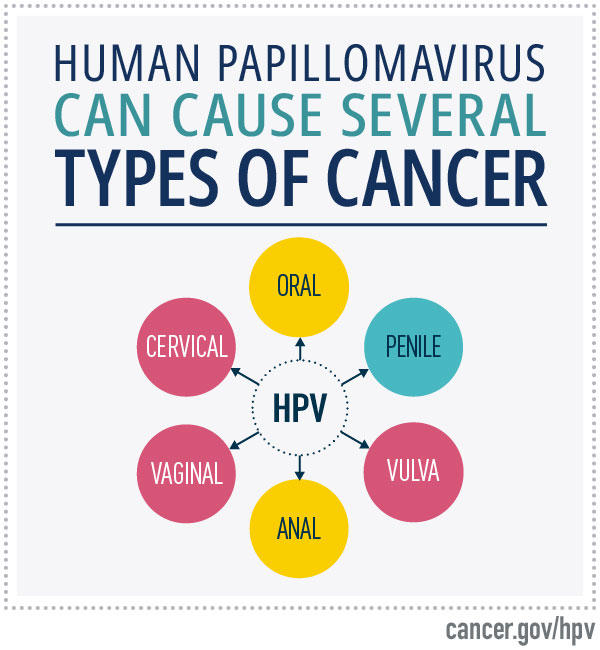
- Human papillomaviruses (HPV), high-risk types of which cause cervical cancer, most anal cancers, and oropharyngeal, penile, vaginal, and vulvar cancer
- Hepatitis B virus (HBV) and hepatitis C virus (HCV), which both cause liver cancer
HIV-infected persons are more likely to be infected with these viruses than people in the general population (10–13).
In addition, the prevalence of some traditional risk factors for cancer, especially smoking (a known cause of lung and other cancers) and heavy alcohol use (which can increase the risk of liver cancer), is higher among people infected with HIV (12, 14). Also, because people infected with HIV have compromised immune systems, both immunosuppression and inflammation may have direct or indirect roles in the development of some cancers that are elevated in people infected with HIV (2, 9).
The poorer cancer survival of HIV-infected people may result, at least in part, from the weakened immune system in such individuals. The increased risk of death could also result from the cancer
Has the introduction of antiretroviral therapy changed the cancer risk of people infected with HIV?
The introduction of highly active antiretroviral therapy (HAART), also called combination antiretroviral therapy (cART), starting in the mid-1990s greatly reduced the incidence of certain cancers in HIV-infected patients, especially Kaposi sarcoma and non-Hodgkin lymphoma (2). The likely explanation for this reduced incidence is that cART lowers the amount of HIV circulating in the blood, thereby allowing partial restoration of immune system function to fight the viruses that cause many of these cancers.
Although the risk of these AIDS-defining cancers among people infected with HIV is lower than in the past, it is still much higher than among people in the general population (15). This persistently high risk may reflect the fact that cART does not completely restore immune system functioning. Also, many people infected with HIV are not aware they are infected, have had difficulty in accessing medical care, or for other reasons are not receiving adequate antiretroviral therapy.
The introduction of cART has not reduced the incidence of all HIV-related cancers, and in
An important factor contributing to the increase in
What can people infected with HIV do to reduce their risk of cancer or to find cancer early?
Taking cART as indicated based on current HIV treatment guidelines lowers the risk of Kaposi sarcoma and non-Hodgkin lymphoma and increases overall survival.
The risk of lung, oral, and other cancers can be reduced by quitting smoking. Because HIV-infected people have a higher risk of lung cancer, it is especially important that they do not smoke. Help with quitting smoking is available through the National Cancer Institute’s (NCI’s) smoking quitline at 1–877–448–7848 (1–877–44U–QUIT) and other NCI resources, which are listed on the Tobacco page.
The higher incidence of liver cancer among HIV-infected people appears to be related to more frequent infection with hepatitis virus (particularly HCV in the United States) than among HIV-uninfected people (12, 16). Therefore, HIV-infected individuals should know their hepatitis status.
In addition, if HIV-infected people currently have viral hepatitis, they should discuss with their
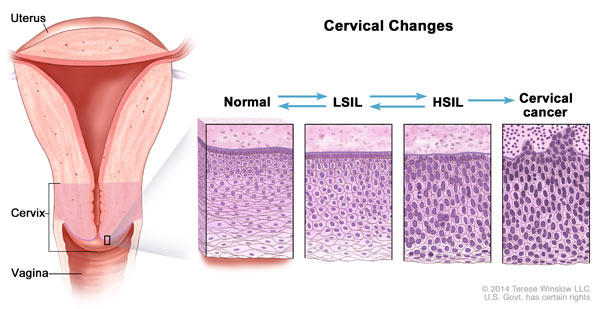
Because HIV-infected women have a higher risk of cervical cancer, it is important that they
Some researchers recommend anal Pap test screening to detect and treat early lesions before they progress to anal cancer (21). However, it is not clear if this type of screening benefits all HIV-infected people or if treating such lesions prevents anal cancer. These questions are being addressed in
KSHV is secreted in saliva, and transmission of this virus may occur through deep kissing, through the use of saliva as a lubricant in sex, or through
How does NCI support research on HIV/AIDS-related cancers?
The Office of HIV and AIDS Malignancy (OHAM) coordinates and oversees NCI-sponsored research on AIDS-related cancers and HIV/AIDS. OHAM also acts as a point of contact for the National Institutes of Health (NIH) Office of AIDS Research (OAR).
OHAM has two programs:
- The AIDS Malignancy Program, which has primary responsibility for identifying new initiatives; for sponsoring international activities, such as the Strengthening Capacity for Research for HIV-Associated Malignancies in Africa initiative; and for overseeing programs that NCI co-manages with other NIH institutes, such as the Centers for AIDS Research, the Multicenter AIDS Cohort Study, the Women’s Interagency HIV Study, and the International Epidemiology Databases to Evaluate AIDS.
- The AIDS Cancer Clinical Program, which has primary responsibility for overseeing clinical programs in OHAM, including the AIDS Malignancy Consortium and the AIDS and Cancer Specimen Resource.
The two intramural divisions of NCI, the Center for Cancer Research (CCR) and the Division of Cancer Epidemiology and Genetics (DCEG), conduct research on both HIV and HIV/AIDS-associated cancer. For example, DCEG is conducting the HIV/AIDS Cancer Match Study, which uses data previously collected by public health agencies to examine cancer risk in people with HIV. Nearly all other NCI divisions, offices, and centers also support HIV/AIDS research.