The FDA on June 29, 2020 approved KEYTRUDA® for the first-line treatment of patients with unresectable or metastatic MicroSatellite Instability-High (MSI-H) or MisMatch Repair deficient (dMMR) Colorectal cancer. KEYTRUDA® is a product of Merck & Co.
Author: RR
PHESGO®
The FDA on June 29, 2020 approved a new fixed dose combination of Pertuzumab, Trastuzumab, and Hyaluronidase–zzxf (PHESGO®) for the following indications:
A) Use in combination with chemotherapy as:
1) Neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early stage breast cancer (either greater than 2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer
2) Adjuvant treatment of patients with HER2-positive early breast cancer at high risk of recurrence.
B) Use in combination with Docetaxel for treatment of patients with HER2-positive metastatic breast cancer (MBC) who have not received prior anti-HER2 therapy or chemotherapy for metastatic disease.
PHESGO® is a product of Genentech, Inc.
KEYTRUDA® (Pembrolizumab)
The FDA on June 24, 2020 approved KEYTRUDA® for patients with recurrent or metastatic cutaneous Squamous Cell Carcinoma (cSCC) that is not curable by surgery or radiation. KEYTRUDA® is a product of Merck & Co., Inc.
XPOVIO® (Selinexor)
The FDA on June 22,2020 granted accelerated approval to XPOVIO® for adult patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL), Not Otherwise Specified, including DLBCL arising from Follicular Lymphoma, after at least 2 lines of systemic therapy. XPOVIO® is a product of Karyopharm Therapeutics.
Late Breaking Abstract – ASCO 2020: Adjuvant Therapy with TAGRISSO® Improves Survival in Early Stage EGFR-Mutated Non Small Cell Lung Cancer
SUMMARY: Lung cancer is the second most common cancer in both men and women and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease. Lung cancer is the leading cause of cancer-related mortality in the United States. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of all lung cancers. Of the three main subtypes of NSCLC, 30% are Squamous Cell Carcinomas (SCC), 40% are Adenocarcinomas and 10% are Large Cell Carcinomas. With changes in the cigarette composition and decline in tobacco consumption over the past several decades, Adenocarcinoma now is the most frequent histologic subtype of lung cancer.
Approximately 10-15% of Caucasian patients and 35-50% of Asian patients with Adenocarcinomas, harbor activating EGFR (Epidermal Growth Factor Receptor) mutations and 90% of these mutations are either Exon 19 deletions or L858R substitution mutation in Exon 21. Approximately 25% of patients with EGFR mutated NSCLC have brain metastases at diagnosis, increasing to approximately 40% within two years of diagnosis. The presence of brain metastases often reduces median survival to less than eight months. EGFR-Tyrosine Kinase Inhibitors (TKIs) such as TARCEVA® (Erlotinib), IRESSA® (Gefitinib) and GILOTRIF® (Afatinib), have demonstrated a 60-70% response rate as monotherapy when administered as first line treatment, in patients with metastatic NSCLC, who harbor the sensitizing EGFR mutations. However, majority of these patients experience disease progression within 9-14 months. This resistance to frontline EGFR TKI therapy has been attributed to the most common, acquired T790M “gatekeeper” point mutation in EGFR, identified in 50-60% of patients.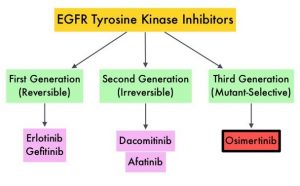
TAGRISSO® (Osimertinib) is a highly selective third-generation Epidermal Growth Factor Receptor (EGFR) TKI presently approved by the FDA, for the first-line treatment of patients with metastatic NSCLC, whose tumors have Exon 19 deletions or Exon 21 L858R mutations, as well as treatment of patients with metastatic EGFR T790M mutation-positive NSCLC, whose disease has progressed on or after EGFR-TKI therapy. Further, TAGRISSO® has higher CNS penetration and is therefore able to induce responses in 70-90% of patients with brain metastases. Among patients with metastatic, EGFR-mutant NSCLC, first-line treatment with TAGRISSO® significantly improved median Overall Survival, compared with TARCEVA® and IRESSA®, and should therefore be considered the preferred regimen.
Surgical resection is the primary treatment for approximately 30% of patients with NSCLC who present with early Stage (I–IIIA) disease. These patients are often treated with Cisplatin-based adjuvant chemotherapy to decrease the risk of recurrence. Nonetheless, 45-75% of these patients develop recurrent disease. There is therefore an unmet need for this patient population.
ADAURA is a global, double-blind, randomized Phase III study, which assessed the efficacy and safety of TAGRISSO® versus placebo in patients with Stage IB–IIIA EGFR mutated NSCLC, after complete tumor resection and adjuvant chemotherapy, when indicated. In this study, 682 patients with completely resected Stage IB, II, IIIA NSCLC, with or without postoperative adjuvant chemotherapy, were randomly assigned 1:1 to receive either TAGRISSO® 80 mg orally once daily (N=339) or placebo (N=343) once daily, for up to 3 years. Eligible patients had an ECOG Performance Status of 0 or 1, with confirmed EGFR mutations (Exon 19del or L858R). Treatment groups were well balanced and patients were stratified by Stage (IB/II/IIIA), mutation type (Exon 19del/L858R), and race (Asian/non-Asian). The Primary endpoint was Disease Free Survival (DFS) in Stage II–IIIA patients. Secondary endpoints included Overall Survival (OS) and safety. Following Independent Data Monitoring Committee recommendation, the trial was unblinded early, due to efficacy. The authors reported the results from the unplanned interim analysis.
It was noted that in the patients with Stage II/IIIA disease, the DFS had not been reached with TAGRISSO® versus 20.4 months with placebo (HR=0.17; P<0.0001). The 2-year DFS rate in this patient group with TAGRISSO® was 90% versus 44% with placebo. In the overall population, the DFS was still not reached with TAGRISSO® versus 28.1 months with placebo (HR=0.21; P<0.0001). The 2-year DFS rate in the overall population was 89% with TAGRISSO® versus 53% with placebo. The OS data are still early and immature, and the median OS has not yet been reached in either treatment groups. The safety profile was consistent with the known safety profile of TAGRISSO®.
The authors concluded that adjuvant TAGRISSO® is the first targeted agent in a global randomized trial, to show a statistically significant and clinically meaningful improvement in Disease Free Survival, among patients with Stage IB/II/IIIA EGFR mutation-positive NSCLC, and provides an effective new treatment strategy for this patient group.
Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. Herbst RS, Tsuboi M, John T, et al. J Clin Oncol 38: 2020 (suppl; abstr LBA5)
KEYTRUDA® Superior to ADCETRIS® in Relapsed or Refractory Classical Hodgkin Lymphoma
SUMMARY: The American Cancer Society estimates that in the United States for 2020, about 8,480 new cases of Hodgkin Lymphoma will be diagnosed and about 970 patients will die of the disease. Hodgkin Lymphoma is classified into two main groups – Classical Hodgkin Lymphomas and Nodular Lymphocyte Predominant type, by the World Health Organization. The Classical Hodgkin Lymphomas include Nodular sclerosing, Mixed cellularity, Lymphocyte rich, Lymphocyte depleted subtypes and accounts for approximately 10% of all malignant lymphomas. Nodular sclerosis Hodgkin Lymphoma histology, accounts for approximately 80% of Hodgkin lymphoma cases in older children and adolescents in the United States. Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells, collectively known as Hodgkin and Reed-Sternberg cells (HRS).The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells.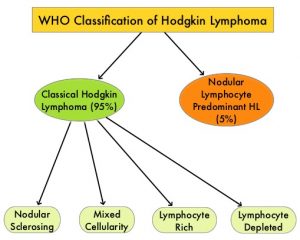
Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-L. Classical Hodgkin Lymphoma is an excellent example of how the tumor microenvironment influences cancer cells to proliferate and survive. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2, with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells, as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin lymphomas. It would therefore seem logical to block or inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2.
Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells. ADCETRIS® (Brentuximab vedotin) is an Antibody-Drug Conjugate (ADC) that targets CD30, which is a surface antigen, expressed on Reed-Sternberg cells, in patients with Classical Hodgkin Lymphoma. This ADC consists of an anti-CD30 monoclonal antibody linked to MonoMethyl Auristatin E (MMAE), an antimicrotubule agent. Upon binding to the CD30 molecule on the cancer cells, MMAE is released into the cancer cell, resulting in cell death.
Patients with Relapsed or Refractory Classical Hodgkin Lymphoma (R/R cHL) are often treated with salvage chemotherapy and Autologous Stem Cell Transplant (ASCT). There are however no standard interventions for patients ineligible for ASCT due to chemo-refractory disease, comorbidity, or advanced stage. PD-1 inhibitor such as KEYTRUDA® as well as ADCETRIS® has shown antitumor activity in R/R cHL.
KEYNOTE-204 is a randomized, international, open-label, Phase III study in which KEYTRUDA® was compared with ADCETRIS® among patients with Relapsed or Refractory Classical Hodgkin Lymphoma (R/R cHL). In this study, 304 patients were randomized 1:1, and 300 patients were treated and assigned to receive KEYTRUDA® 200 mg IV every 3 weeks (N=148) or ADCETRIS® 1.8 mg/kg IV every 3 weeks (N=152). Enrolled patients were post-Autologous Stem Cell Transplant (ASCT) or ineligible for ASCT, had measurable disease and had an ECOG Performance Status of 0 or 1. Both ADCETRIS®-naive and ADCETRIS®-exposed patients were eligible. Patients were stratified by prior ASCT and status after first-line therapy (primary refractory versus relapsed less than 12 months versus relapsed 12 months or more after end of first-line therapy). The Primary endpoints were Progression Free Survival (PFS) per Blinded Independent Central Review (BICR) and Overall Survival (OS). Secondary endpoints included PFS per investigator review, Objective Response Rate (ORR), and Safety. Median follow up was 24.7 months.
The median PFS was 13.2 months in the KEYTRUDA® group compared with 8.3 months in the ADCETRIS® group (HR=0.65, P=0.00271), suggesting an increase in PFS of 4.9 months with KEYTRUDA®. This benefit with KEYTRUDA® was observed in all subgroups tested, including those ineligible for ASCT (HR=0.61), those with primary refractory disease (HR=0.52), those who were ADCETRIS® naïve (HR=0.67), as well as those who received prior treatment with ADCETRIS® (HR=0.34). The ORR was 65.6% versus 54.2%, and the median Duration of Response was 20.7 months versus 13.8 months, in the KEYTRUDA® and ADCETRIS® groups respectively. Treatment Related Adverse Events were similar in both treatment groups and Grade 3-5 toxicities occurred in 19.6% of patients treated with KEYTRUDA® and 25% of patients treated with ADCETRIS®.
It was concluded that among patients with Relapsed/Refractory Classical Hodgkin Lymphoma, KEYTRUDA® was superior to ADCETRIS®, with a statistically significant and clinically meaningful improvement in PFS across all subgroups tested, and with safety consistent with previous reports. The authors added that KEYTRUDA® should be considered the preferred treatment option and the new standard of care in this patient population.
KEYNOTE-204: Randomized, open-label, phase III study of pembrolizumab (pembro) versus brentuximab vedotin (BV) in relapsed or refractory classic Hodgkin lymphoma (R/R cHL). Kuruvilla J, Ramchandren R, Santoro A, et al. Presented at: 2020 ASCO Virtual Scientific Program; May 29, 2020. J Clin Oncol 38: 2020 (suppl; abstr 8005).
TAZVERIK® (Tazemetostat)
The FDA on June 18, 2020 granted accelerated approval to TAZVERIK®, an EZH2 inhibitor, for adult patients with Relapsed or Refractory (R/R) Follicular Lymphoma (FL) whose tumors are positive for an EZH2 mutation, as detected by an FDA-approved test, and who have received at least 2 prior systemic therapies, and for adult patients with R/R FL who have no satisfactory alternative treatment options. TAZVERIK® is a product of Epizyme, Inc.
KEYTRUDA® (Pembrolizumab)
The FDA on June 16, 2020 granted accelerated approval to KEYTRUDA® for the treatment of adult and pediatric patients with unresectable or metastatic Tumor Mutational Burden-High (TMB-H) [10 or more mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options. KEYTRUDA® is a product of Merck & Co., Inc.
ZEPZELCA® (Lurbinectedin)
The FDA on June 15, 2020 granted accelerated approval to ZEPZELCA® for adult patients with metastatic Small Cell Lung Cancer (SCLC), with disease progression on or after platinum-based chemotherapy. ZEPZELCA® is a product of Pharma Mar S.A.
OPDIVO® (Nivolumab)
The FDA on June 10, 2020 approved OPDIVO® for patients with unresectable, advanced, recurrent or metastatic Esophageal Squamous Cell Carcinoma (ESCC), after prior Fluoropyrimidine- and Platinum-based chemotherapy. OPDIVO® is a product of Bristol-Myers Squibb Co.
