Special Written by Dr. Robert Rifkin, Rocky Mountain Cancer Center | Sponsored by Adaptive Biotechnologies
Rising Importance of MRD Testing in Multiple Myeloma
In the early 2000s, the average overall survival rate for patients with multiple myeloma (MM) was only 3 years.1 With the advent of new therapies over the last 5 years, many patients with MM can now expect to achieve clinical complete response (CR). However, while this trend is expected to continue, the majority of these patients who achieve CR will eventually relapse, suggesting that existing therapies are insufficient and more sensitive testing is necessary to identify potentially undetected malignant cells.2
Minimal residual disease (MRD) refers to the small number of cancer cells that can remain in a patient’s body during and after treatment and may eventually cause recurrence of the disease. MRD is commonly assessed in lymphoid malignancies such as B-cell acute lymphoblastic leukemia (B-ALL), chronic lymphocytic leukemia (CLL) and multiple myeloma (MM). In the event of the persistence of malignant B cells, the possibility of recurrence is more likely.3 To address this, MRD testing is now being used to monitor the effectiveness of therapies as well as subsequent treatment decisions by identifying the presence of MRD over time.
The Application of Next-Generation Sequencing
MRD testing in lymphoid malignancies has become increasingly valuable in predicting patient outcomes. While next-generation flow cytometry has been used for MRD testing in B-ALL, and has been standardized for highly sensitive MRD measurements (e.g. 10-6), as reported by Theunissen and Colleagues, standard flow cytometry is limited to a level of detection of 1 malignant cell in 10,000 cells assessed (e.g. 10-4)4. In contrast, next-generation sequencing (NGS) has a level of sensitivity of up to 1 malignant cell in 1,000,000 cells assessed (e.g. 10-6). 5,6
In the era of NGS, it is now possible to assess MRD beyond the standard response criteria for assessment of treatment efficacy. In a review that evaluated the prognostic value of MRD, patients who were MRD negative had a higher probability of prolonged progression-free survival than patients with detectable residual disease, regardless of initial treatment.7
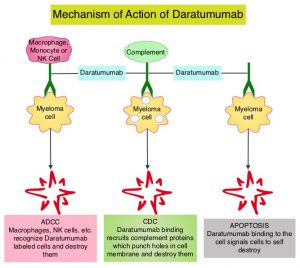
The clonoSEQ® Assay, an in vitro diagnostic (IVD) test that uses multiplex PCR and NGS to identify and quantify disease-associated DNA sequence rearrangements (or clonotypes) of the IgH, IgK and IgL receptor genes, has been FDA-cleared to monitor MRD in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). The assay can accurately and precisely quantify MRD at the DNA-sequence level. According to a recent analysis, clonoSEQ maintains accurate reporting of disease burden down to one malignant cell in 1 million healthy cells provided sufficient sample input.5,6
Patient-specific clonal sequences are identified at the time of diagnosis or high disease burden and can be used as a marker for MRD. Oftentimes, at the conclusion of therapy, MRD measurements can also be used to firmly establish a diagnosis of a molecular complete remission. In order to do this with an NGS assay, it is important to remember to obtain a baseline fresh bone marrow sample at the time of diagnosis. This will facilitate the identification of a dominant clone. In the event such a sample is not available, it is possible to identify the clone utilizing archived or fixed tissue.
Incorporating MRD Testing in Clinical Practice Guidelines
The future of MRD testing in MM, as reviewed by Oliva and colleagues, is clear: MRD testing in MM will be increasingly important as we strive for a cure.8 The course of MM is highly variable, and the clinical behavior is equally diverse. For this reason, MRD testing has been incorporated into clinical practice guidelines as a Standard of Care, as evidenced by the NCCN’s recommendation to assess MRD after each stage of treatment: post-induction, post-high-dose therapy/ASCT, post-consolidation, post-maintenance. NCCN updated their guidelines recently to note that during upfront diagnosis you could consider “baseline clone identification and storage of aspirate samples for future MRD testing by NGS”.9
In short, MRD testing in lymphoid malignancies should be leveraged to track a patient’s disease over time. This approach may aid in key clinical decision-making throughout the course of treatment. For example, if MRD is present in a B-ALL patient, therapy with blinatumomab is suggested over other agents and is now part of guidelines. If MRD is negative, alternative maintenance with the POMP regimen is often employed. Similar guidelines for MM and CLL are on the therapeutic horizon, and I suspect will soon be incorporated into evidence-based guidelines.
As we enter the new area of targeted therapy and the development of novel agents for all the diseases, testing for MRD will become increasingly important. In order to maintain a state-of-the-art clinical practice, and to foster best clinical practice in patient care, it essential that every clinician and stakeholder in the patient’s journey become familiar with these new MRD technologies, and how to integrate them into his or her overall care plan in order to improve clinical outcomes.
Important information
clonoSEQ is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect measurable residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). clonoSEQ is also available for use in other lymphoid cancers as a CLIA-validated laboratory developed test (LDT) service. For important information about the FDA-cleared uses of clonoSEQ including test limitations, please visit https://www.clonoseq.com/technical-summary/.
References
1) Landgren O, Iskander K. J Intern Med. 2017;281(4):365-382.
2) Munshi NC, Anderson KC. J Clin Oncol. 2013;31 (20):2523-2526.
3) Perrot A, Lauwers-Cances V, Corre J, et al. Blood. 2018;132(23):2456-2464.
4) Theunissen P, Mejstrikova E, et al. Blood. (2017) 129 (3): 347–357.
5) clonoSEQ®. [technical summary]. Seattle, WA: Adaptive Biotechnologies; 2020.
6) Ching T, Duncan ME, et al. BMC Cancer. 2020; 20: 612.
7) Rajkumar SV, Kumar S. Mayo Clin Proc. 2016 Jan;91(1):101-19.
8) Oliva S, D’Agostino M, et al. Front Oncol. 2020; 10: 1.
9) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.1.2020. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed March September 22nd, 2020. To view the most recent and complete version of the guideline, go to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use of application and disclaims any responsibility for their application or use in any way.

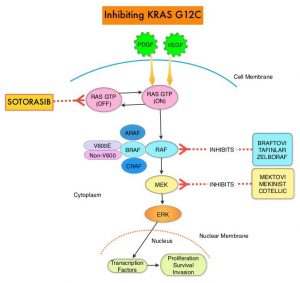
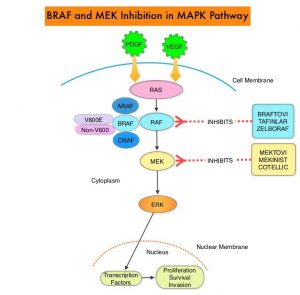
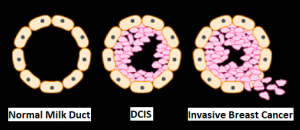
 and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease.
and accounts for about 14% of all new cancers and 27% of all cancer deaths. The American Cancer Society estimates that for 2020, about 228, 820 new cases of lung cancer will be diagnosed and 135,720 patients will die of the disease.